Covarrubias 2021 Nat Rev Mol Cell Biol
| Covarrubias AJ, Perrone R, Grozio A, Verdin E (2021) NAD+ metabolism and its roles in cellular processes during ageing. Nat Rev Mol Cell Biol 22:119-41. https://doi.org/10.1038/s41580-020-00313-x |
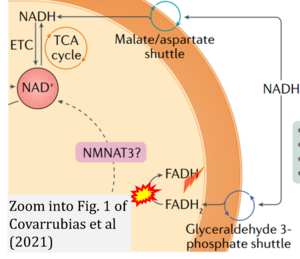
Covarrubias AJ, Perrone R, Grozio A, Verdin E (2021) Nat Rev Mol Cell Biol
Abstract: Nicotinamide adenine dinucleotide (NAD+) is a coenzyme for redox reactions, making it central to energy metabolism. NAD+ is also an essential cofactor for non-redox NAD+-dependent enzymes, including sirtuins, CD38 and poly(ADP-ribose) polymerases. NAD+ can directly and indirectly influence many key cellular functions, including metabolic pathways, DNA repair, chromatin remodelling, cellular senescence and immune cell function. These cellular processes and functions are critical for maintaining tissue and metabolic homeostasis and for healthy ageing. Remarkably, ageing is accompanied by a gradual decline in tissue and cellular NAD+ levels in multiple model organisms, including rodents and humans. This decline in NAD+ levels is linked causally to numerous ageing-associated diseases, including cognitive decline, cancer, metabolic disease, sarcopenia and frailty. Many of these ageing-associated diseases can be slowed down and even reversed by restoring NAD+ levels. Therefore, targeting NAD+ metabolism has emerged as a potential therapeutic approach to ameliorate ageing-related disease, and extend the human healthspan and lifespan. However, much remains to be learnt about how NAD+ influences human health and ageing biology. This includes a deeper understanding of the molecular mechanisms that regulate NAD+ levels, how to effectively restore NAD+ levels during ageing, whether doing so is safe and whether NAD+ repletion will have beneficial effects in ageing humans.
• Bioblast editor: Gnaiger E
Correction: FADH2 and Complex II
- FADH2 is shown as the substrate feeding electrons into Complex II (CII). This is wrong and requires correction - for details see Gnaiger (2024).
- Gnaiger E (2024) Complex II ambiguities ― FADH2 in the electron transfer system. J Biol Chem 300:105470. https://doi.org/10.1016/j.jbc.2023.105470 - »Bioblast link«
- FADH2/FAD does not reach the mt-matrix in the reaction catalyzed by mitochondrial glycerophosphate dehydrogenase. Nevertheless, FADH should be corrected to FAD.
- Gnaiger E (2024) Complex II ambiguities ― FADH2 in the electron transfer system. J Biol Chem 300:105470. https://doi.org/10.1016/j.jbc.2023.105470 - »Bioblast link«
Labels:
NAD