Kikusato 2016 Proc Jpn Soc Anim Nutr Metab
| Kikusato M, Furukawa K, Kamizono T, Hakamata Y, Toyomizu M (2016) Roles of mitochondrial oxidative phosphorylation and reactive oxygen species generation in the metabolic modification of avian skeletal muscle. Proc Jpn Soc Anim Nutr Metab 60:57-68. |
Kikusato M, Furukawa K, Kamizono, Hakamata Y, Toyomizu M (2016) Proc Jpn Soc Anim Nutr Metab
Abstract: Mitochondria are double membrane-bound organelles found in most eukaryotic cells and serve as the centers of intracellular metabolism. One of their major functions is ATP production by oxidative phosphorylation (OXPHOS). OXPHOS is an energy transduction system that is based on coupling NADH/FADH2 oxidation-driven electron transfer with ADP phosphorylation, with the inner membrane potential (ΔΨ) serving as a mediator. The coupling efficiency of OXPHOS contributes to the growth rate of chickens. The mechanistic understanding of this efficiency is therefore quite important. Herein, we review the general mechanisms underlying OXPHOS, and describe modular kinetic analysis, which is the methodology used for determining OXPHOS efficiency. Moreover, this review describes our experimental adaptation of the kinetic method to avian muscle mitochondria. We show the analysis for the differences in the coupling efficiency between meat- and laying-type chickens and the implication of these differences in determining the growth rate in these birds. The review also discusses the role of mitochondria as a major intracellular reactive oxygen species (ROS) generator and their effects on cellular metabolism. Mitochondria-generated ROS cause oxidative disturbance in cells, and participate in intracellular signal transduction pathways evoking cell death and protein catabolism, in response to several physiological and pathological stimuli. We describe that heat stress (HS) stimulates mitochondrial ROS generation, which may be caused by OXPHOS alterations and the subsequent increase in ΔΨ. Finally, we suggest that HS-induced mitochondrial ROS generation may play an important role in the induction of ubiquitin-proteasome-dependent protein degradation in avian skeletal muscle, which is partially responsible for growth retardation in the HS-treated birds.
• Bioblast editor: Gnaiger E
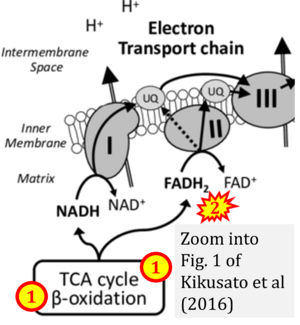
Correction: FADH2 and Complex II
- FADH2 is shown as the substrate feeding electrons into Complex II (CII). This is wrong and requires correction - for details see Gnaiger (2024).
- Gnaiger E (2024) Complex II ambiguities ― FADH2 in the electron transfer system. J Biol Chem 300:105470. https://doi.org/10.1016/j.jbc.2023.105470 - »Bioblast link«