Vorotnikov 2022 Biomedicines
| Vorotnikov AV, Khapchaev AY, Nickashin AV, Shirinsky VP (2022) In vitro modeling of diabetes impact on vascular endothelium: Are essentials engaged to tune metabolism? Biomedicines 10:3181. https://doi.org/10.3390/biomedicines10123181 |
Vorotnikov AV, Khapchaev AY, Nickashin AV, Shirinsky VP (2022) Biomedicines
Abstract: Angiopathy is a common complication of diabetes mellitus. Vascular endothelium is among the first targets to experience blood-borne metabolic alterations, such as hyperglycemia and hyperlipidemia, the hallmarks of type 2 diabetes. To explore mechanisms of vascular dysfunction and eventual damage brought by these pathologic conditions and to find ways to protect vasculature in diabetic patients, various research approaches are used including in vitro endothelial cell-based models. We present an analysis of the data available from these models that identifies early endothelial cell apoptosis associated with oxidative stress as the major outcome of mimicking hyperglycemia and hyperlipidemia in vitro. However, the fate of endothelial cells observed in these studies does not closely follow it in vivo where massive endothelial damage occurs mainly in the terminal stages of diabetes and in conjunction with comorbidities. We propose that the discrepancy is likely in missing essentials that should be available to cultured endothelial cells to adjust the metabolic state and withstand the immediate apoptosis. We discuss the role of carnitine, creatine, and AMP-activated protein kinase (AMPK) in suiting the endothelial metabolism for long-term function in diabetic type milieu in vitro. Engagement of these essentials is anticipated to expand diabetes research options when using endothelial cell-based models.
• Bioblast editor: Gnaiger E
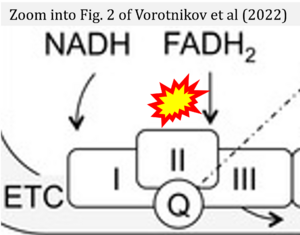
Correction: FADH2 and Complex II
- FADH2 is shown as the substrate feeding electrons into Complex II (CII). This is wrong and requires correction - for details see Gnaiger (2024).
- Gnaiger E (2024) Complex II ambiguities ― FADH2 in the electron transfer system. J Biol Chem 300:105470. https://doi.org/10.1016/j.jbc.2023.105470 - »Bioblast link«
Labels:
Enzyme: Complex II;succinate dehydrogenase