Acuna-Castroviejo 2012 Abstract Bioblast: Difference between revisions
Marte Verena (talk | contribs) m (Reverted edits by Marte Verena (talk) to last revision by Meissner Barbara) |
No edit summary |
||
| Line 24: | Line 24: | ||
|enzymes=Complex I, Complex II; Succinate Dehydrogenase, Complex III, Complex V; ATP Synthase | |enzymes=Complex I, Complex II; Succinate Dehydrogenase, Complex III, Complex V; ATP Synthase | ||
|kinetics=ADP; Pi, Oxygen | |kinetics=ADP; Pi, Oxygen | ||
|topics=Flux Control; Threshold; Excess Capacity, Membrane | |topics=ATP; ADP; AMP; PCr, Flux Control; Threshold; Excess Capacity, mt-Membrane potential, Redox state | ||
|journal=Mitochondr Physiol Network | |journal=Mitochondr Physiol Network | ||
|articletype=Abstract | |articletype=Abstract | ||
Revision as of 16:52, 6 August 2013
| Has title::Acuna-Castroviejo D (2012) Melatonin and the quality of life: the melatonin-mitochondrion connection. Mitochondr Physiol Network 17.12. |
Link: MiPNet17.12 Bioblast 2012 - Open Access
Was written by::Acuna-Castroviejo D (Was submitted in year::2012)
Event: [[Was submitted to event::Bioblast 2012]]
[[has abstract::
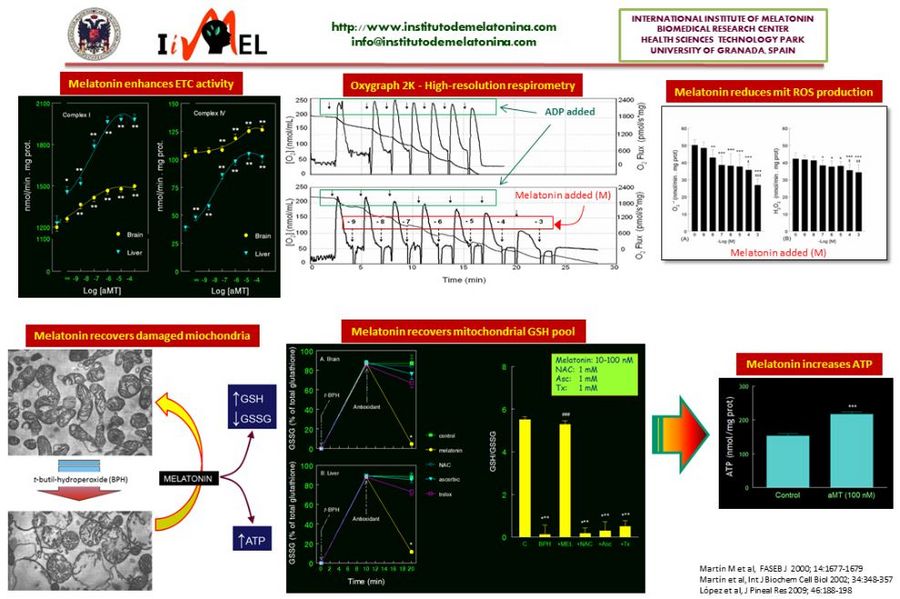
The first relationship between melatonin and mitochondria came from histological studies showing changes in mitochondrial density and morphology after pinealectomy or melatonin administration to experimental animals. After the discovery of the antioxidant activity of melatonin in 1993, the possibility that melatonin exerts its effects on the mitochondria, the main ROS-producing organelle, was hypothesized. The first experiments demonstrated a highly efficient ability of melatonin to counteract the mitochondrial oxidative stress in vitro and in vivo. In parallel, melatonin increases the respiratory chain activity, reduces the oxygen consumption, and increases the ATP production. In some of these experiments we could demonstrated that the mitochondria take up melatonin in a time- and concentration-dependent manner. To further analyze the ability of melatonin to prevent and/or counteract mitochondrial dysfunction, different experimental models of aging and disease, including sepsis, Parkinson’s disease, and Alzheimer’s disease, was evaluated. In all of them, melatonin administration restored the full bioenergetic capacity of the mitochondria, restoring or even increasing their ATP production. Along this time, it was showed that most of the tissues and organs produce melatonin independently of the pineal gland. An important feature of this extrapineal source of melatonin is that its synthetizing enzymes, AANAT and ASMT, are inducibles, i.e., the cells produce melatonin when they require it for protective purposes. This melatonin does not exit to the extracellular fluid. To further analyze the dynamics of the extrapineal melatonin, we recently studied the subcellular distribution of the indoleamine in liver and brain. These studies showed that melatonin is produced in considerably higher amounts in these tissues than in the pineal gland, it is not uniformly distributed in the cell, and is mainly located in the membrane, mitochondria and nucleus. Interestingly, membrane content of melatonin increased in a dose-dependent manner after administration of melatonin to rats, but the content of the indoleamine in the nucleus and mitochondria is saturated. There are now evidences of the ability of mitochondria (and chloroplasts) to synthesize melatonin, which explains the high levels of the indoleamine in this organelle. The phylogenetic origins of the mitochondria, and the presence of melatonin in ancient one cell organisms, speak in favor of a melatonin-mitochondrion connection along the evolution, and the role of melatonin in mitochondrial homeostasis.
- Acuna Castroviejo D, Lopez LC, Escames G, Lopez A, Garcia JA, Reiter RJ (2011) Melatonin-mitochondria interplay in health and disease. Curr Top Med Chem 11: 221-240.
- Tan DX, Manchester LC, Liu X, Rosales-Corral SA, Acuna-Castroviejo D, Reiter RJ (2012) Mitochondria and chloroplasts as the original sites of melatonin synthesis: a hypothesis related to melatonin's primary function and evolution in eukaryotes. J Pineal Res. doi: 10.1111/jpi.12026]]
• Keywords: has publicationkeywords::Melatonin, has publicationkeywords::Mitochondria, has publicationkeywords::Reactive oxygen species, has publicationkeywords::Respiratory chain, has publicationkeywords::ATP
• O2k-Network Lab: Was published by MiPNetLab::ES Granada Acuna-Castroviejo D
Labels:
Stress:Injury and adaptation::RONS; Oxidative Stress, Injury and adaptation::Mitochondrial Disease; Degenerative Disease and Defect, Injury and adaptation::Aging; Senescence Organism: Organism::Mouse, Organism::Rat Tissue;cell: tissue and cell::Heart, tissue and cell::Skeletal muscle, tissue and cell::Nervous system, tissue and cell::Liver Preparation: Preparation::Intact Organism, Preparation::Isolated Mitochondria Enzyme: Enzyme::Complex I, Enzyme::Complex II; Succinate Dehydrogenase, Enzyme::Complex III, Enzyme::Complex V; ATP Synthase Regulation: Topic::ATP; ADP; AMP; PCr, Topic::Flux Control; Threshold; Excess Capacity, Topic::mt-Membrane potential, Topic::Redox state Coupling state: Coupling states::ROUTINE, Coupling states::OXPHOS
HRR: Instrument and method::Oxygraph-2k
Affiliations and author contributions
Instituto de Biotecnología, Centro de Investigación Biomédica, Parque Tecnológico de Ciencias de la Salud, Universidad de Granada, Granada, Spain. E-mail: [email protected]
Supported by grants # P10-CTS-5784 and PI08-1664.