Keidar 2023 Front Physiol
| Keidar N, Peretz NK and Yaniv Y (2023) Ca2+ pushes and pulls energetics to maintain ATP balance in atrial cells: computational insights. Front Physiol 14:1231259. https://doi.org/10.3389/fphys.2023.1231259 |
Keidar N, Peretz NK and Yaniv Y (2023) Front Physiol
Abstract: To maintain atrial function, ATP supply-to-demand matching must be tightly controlled. Ca 2+ can modulate both energy consumption and production. In light of evidence suggesting that Ca 2+ affects energetics through “push” (activating metabolite flux and enzymes in the Krebs cycle to push the redox flux) and “pull” (acting directly on ATP synthase and driving the redox flux through the electron transport chain and increasing ATP production) pathways, we investigated whether both pathways are necessary to maintain atrial ATP supply-to-demand matching. Rabbit right atrial cells were electrically stimulated at different rates, and oxygen consumption and flavoprotein fluorescence were measured. To gain mechanistic insight into the regulators of ATP supply-to-demand matching in atrial cells, models of atrial electrophysiology, Ca 2+ cycling and force were integrated with a model of mitochondrial Ca 2+ and a modified model of mitochondrial energy metabolism. The experimental results showed that oxygen consumption increased in response to increases in the electrical stimulation rate. The model reproduced these findings and predicted that the increase in oxygen consumption is associated with metabolic homeostasis. The model predicted that Ca 2+ must act both in “push” and “pull” pathways to increase oxygen consumption. In contrast to ventricular trabeculae, no rapid time-dependent changes in mitochondrial flavoprotein fluorescence were measured upon an abrupt change in workload. The model reproduced these findings and predicted that the maintenance of metabolic homeostasis is due to the effects of Ca 2+ on ATP production. Taken together, this work provides evidence of Ca 2+ “push” and “pull” activity to maintain metabolic homeostasis in atrial cells.
Labels: MiParea: Respiration
Enzyme: Complex II;succinate dehydrogenase Regulation: Calcium
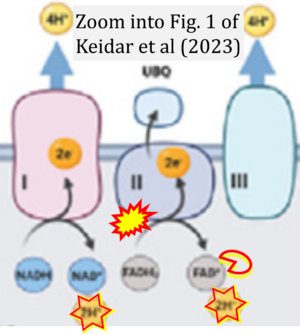
Correction: FADH2 and Complex II
- FADH2 is shown as the substrate feeding electrons into Complex II (CII). This is wrong and requires correction - for details see Gnaiger (2024).
- Gnaiger E (2024) Complex II ambiguities ― FADH2 in the electron transfer system. J Biol Chem 300:105470. https://doi.org/10.1016/j.jbc.2023.105470 - »Bioblast link«