Difference between revisions of "Koopman 2016 Nat Protoc"
(Created page with "{{Publication |title=Koopman M, Michels H, Dancy BM, Kamble R, Mouchiroud L, Auwerx J, Nollen EA, Houtkooper RH (2016) A screening-based platform for the assessment of cellula...") |
|||
| Line 14: | Line 14: | ||
|instruments=Oxygraph-2k | |instruments=Oxygraph-2k | ||
}} | }} | ||
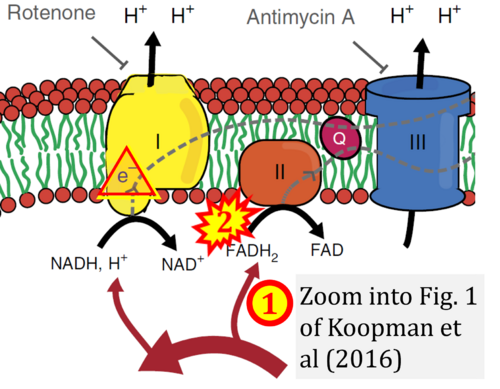
[[File:Koopman 2016 Nat Protoc CORRECTION.png|right|500px]] | |||
{{Template:Correction FADH2 and S-pathway}} | |||
Revision as of 10:59, 2 August 2023
| Koopman M, Michels H, Dancy BM, Kamble R, Mouchiroud L, Auwerx J, Nollen EA, Houtkooper RH (2016) A screening-based platform for the assessment of cellular respiration in Caenorhabditis elegans. Nat Protoc 11:1798-816. |
Koopman M, Michels H, Dancy BM, Kamble R, Mouchiroud L, Auwerx J, Nollen EA, Houtkooper RH (2016) Nat Protoc
Abstract: Mitochondrial dysfunction is at the core of many diseases, ranging from inherited metabolic diseases to common conditions that are associated with ageing. While associations between ageing and mitochondrial function have been identified using mammalian models, much of the mechanistic insight has emerged from C. elegans. Mitochondrial respiration is recognized as an indicator of mitochondrial health. Seahorse XF96 respirometers are the state-of-the-art platform to assess respiration in cells, and we adapted the technique for applications involving C. elegans. Here, we provide a detailed protocol to optimise and measure respiration in C. elegans with the XF96 respirometer, including the interpretation of parameters and results. The protocol takes ~2 days to complete, excluding time spent culturing C. elegans, and includes (i) the preparation of C. elegans samples, (ii) selection and loading of compounds to be injected, (iii) preparing and executing a run with the XF96 respirometer, and (iv) post-experimental data-analysis, including normalization. In addition, we compare our XF96 application with other existing techniques, including the 8-well Seahorse XFp. The main benefits of the XF96 include the limited number of worms required and high-throughput capacity due to 96-well format.
• Bioblast editor: Gnaiger E
Labels: MiParea: Respiration
Organism: Caenorhabditis elegans, Nematodes
Preparation: Intact organism
HRR: Oxygraph-2k
Correction: FADH2 and Complex II
- FADH2 is shown as the substrate feeding electrons into Complex II (CII). This is wrong and requires correction - for details see Gnaiger (2024).
- Gnaiger E (2024) Complex II ambiguities ― FADH2 in the electron transfer system. J Biol Chem 300:105470. https://doi.org/10.1016/j.jbc.2023.105470 - »Bioblast link«