Reiss 2022 Exp Gerontol
| Reiss AB, Ahmed S, Dayaramani C, Glass AD, Gomolin IH, Pinkhasov A, Stecker MM, Wisniewski T, De Leon J (2022) The role of mitochondrial dysfunction in Alzheimer's disease: A potential pathway to treatment. Exp Gerontol 164:111828. https://doi.org/10.1016/j.exger.2022.111828 |
» [https://pubmed.ncbi.nlm.nih.gov/35508280/ PMID: 35508280
Reiss AB, Ahmed S, Dayaramani C, Glass AD, Gomolin IH, Pinkhasov A, Stecker MM, Wisniewski T, De Leon J (2022) Exp Gerontol
Abstract: Background: Alzheimer's disease (AD) is the most prevalent form of dementia worldwide and is characterized by progressive memory loss and cognitive impairment. Our understanding of AD pathogenesis is limited and no effective disease-modifying treatment is available. Mitochondria are cytoplasmic organelles critical to the homeostatic regulation of glucose and energy in the cell.
Methods: Mitochondrial abnormalities are found early in the course of AD and dysfunctional mitochondria are involved in AD progression. The resulting respiratory chain impairment, neuronal apoptosis, and generation of reactive oxygen species are highly damaging to neurons. Restoration of mitochondrial function may provide a novel therapeutic strategy for AD.
Results: This review discusses the specifics of mitochondrial fragmentation, imbalances in fission and fusion, and DNA damage seen in AD and the contribution of compromised mitochondrial activity to AD etiopathogenesis. It explores how an understanding of the processes underlying mitochondrial failure may lead to urgently needed treatment innovations. It considers individual mitochondrial proteins that have emerged as promising drug targets and evaluates neuroprotective agents that could improve the functional state of mitochondria in the setting of AD.
Conclusions: There is great promise in exploring original approaches to preserving mitochondrial viability as a means to achieve breakthroughs in treating AD.
• Bioblast editor: Gnaiger E
Labels:
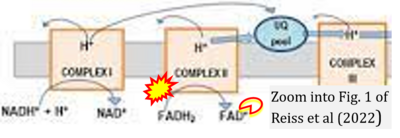
Correction: FADH2 and Complex II
- FADH2 is shown as the substrate feeding electrons into Complex II (CII). This is wrong and requires correction - for details see Gnaiger (2024).
- Gnaiger E (2024) Complex II ambiguities ― FADH2 in the electron transfer system. J Biol Chem 300:105470. https://doi.org/10.1016/j.jbc.2023.105470 - »Bioblast link«