Semantic search
| Term | Abbreviation | Description |
|---|---|---|
| Proton leak | Flux of protons driven by the protonmotive force across the inner mt-membrane, bypassing the ATP synthase and thus contributing to LEAK respiration. Proton leak-flux depends non-linearly (non-ohmic) on the protonmotive force. Compare: Proton slip. | |
| Proton pump | Mitochondrial proton pumps are large enzyme complexes (CI, CIII, CIV, CV) spanning the inner mt-membrane, partially encoded by mtDNA. CI, CIII and CIV are proton pumps that drive protons against the electrochemical protonmotive force, driven by electron transfer from reduced substrates to oxygen. In contrast, ATP synthase (also known as CIV) is a proton pump that utilizes the energy of proton flow along the protonmotive force to drive phosphorylation of ADP to ATP. | |
| Proton slip | Proton slip is a property of the proton pumps (Complexes CI, CIII, and CIV) when the proton slips back to the matrix side within the proton pumping process. Slip is different from the proton leak, which depends on Δp and is a property of the inner mt-membrane (including the boundaries between membrane-spanning proteins and the lipid phase). Slip is an uncoupling process that depends mainly on flux and contributes to a reduction in the biochemical coupling efficiency of ATP production and oxygen consumption. Together with proton leak and cation cycling, proton slip is compensated for by LEAK respiration or LEAK oxygen flux, L. Compare: Proton leak. | |
| Q calibration - DatLab | Q calibration | |
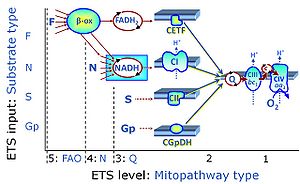
| Q-junction |
The Q-junction is a junction for convergent electron flow in the Electron transfer pathway (ET-pathway) from type N substrates and mt-matrix dehydrogenases through Complex I (CI), from type F substrates and FA oxidation through electron-transferring flavoprotein Complex (CETF), from succinate (S) through Complex II (CII), from glycerophosphate (Gp) through glycerophosphate dehydrogenase Complex (CGpDH), from choline through choline dehydrogenase, from dihydro-orotate through dihydro-orotate dehydrogenase, and other enzyme Complexes into the Q-cycle (ubiquinol/ubiquinone), and further downstream to Complex III (CIII) and Complex IV (CIV). The concept of the Q-junction, with the N-junction and F-junction upstream, provides the rationale for defining Electron-transfer-pathway states and categories of SUIT protocols. | |
| ROUTINE respiration | R | |
| Respiratory acceptor control ratio | RCR | The respiratory acceptor control ratio (RCR) is defined as State 3/State 4 [1]. If State 3 is measured at saturating [ADP], RCR is the inverse of the OXPHOS control ratio, L/P (when State 3 is equivalent to the OXPHOS state, P). RCR is directly but non-linearly related to the P-L control efficiency, jP-L = 1-L/P, with boundaries from 0.0 to 1.0. In contrast, RCR ranges from 1.0 to infinity, which needs to be considered when performing statistical analyses. In living cells, the term RCR has been used for the ratio State 3u/State 4o, i.e. for the inverse L/E ratio [2,3]. Then for conceptual and statistical reasons, RCR should be replaced by the E-L coupling efficiency, 1-L/E [4]. |
| Respiratory chain | RC | The mitochondrial respiratory chain (RC) consists of enzyme complexes arranged to form a metabolic system of convergent pathways for oxidative phosphorylation. In a general sense, the RC includes (1) the electron transfer pathway (ET-pathway), with transporters for the exchange of reduced substrates across the inner mitochondrial membrane, enzymes in the matrix space (particularly dehydrogenases of the tricarboxylic acid cycle), inner membrane-bound electron transfer complexes, and (2) the inner membrane-bound enzymes of the phosphorylation system. |
| Respiratory complexes | CI, CII, CIII, CIV, CETF, CGpDH, .. | Respiratory Complexes are membrane-bound enzymes consisting of several subunits which are involved in energy transduction of the respiratory system. » MiPNet article |
| Respiratory state | Respiratory states of mitochondrial preparations and living cells are defined in the current literature in many ways and with a diversity of terms. Mitochondrial respiratory states must be defined in terms of both, the coupling-control state and the electron-transfer-pathway state. | |
| Respirometry | Respirometry is the quantitative measurement of respiration. Respiration is therefore a combustion, a very slow one to be precise (Lavoisier and Laplace 1783). Thus the basic idea of using calorimetry to explore the sources and dynamics of heat changes were present in the origins of bioenergetics (Gnaiger 1983). Respirometry provides an indirect calorimetric approach to the measurement of metabolic heat changes, by measuring oxygen uptake (and carbon dioxide production and nitrogen excretion in the form of ammonia, urea, or uric acid) and converting the oxygen consumed into an enthalpy change, using the oxycaloric equivalent. Liebig (1842) showed that the substrate of oxidative respiration was protein, carbohydrates, and fat. The sum of these chemical changes of materials under the influence of living cells is known as metabolism (Lusk 1928). The amount (volume STP) of carbon dioxide expired to the amount (volume STP) of oxygen inspired simultaneously is the respiratory quotient, which is 1.0 for the combustion of carbohydrate, but less for lipid and protein. Voit (1901) summarized early respirometric studies carried out by the Munich school on patients and healthy controls, concluding that the metabolism in the body was not proportional to the combustibility of the substances outside the body, but that protein, which burns with difficulty outside, metabolizes with the greatest ease, then carbohydrates, while fats, which readily burns outside, is the most difficultly combustible in the organism. Extending these conclusions on the sources of metabolic heat changes, the corresponding dynamics or respiratory control was summarized (Lusk 1928): The absorption of oxygen does not cause metabolism, but rather the amount of the metabolism determines the amount of oxygen to be absorbed. .. metabolism regulates the respiration. | |
| STPD | STPD | At standard temperature and pressure dry (STPD: 0 °C = 273.15 K and 1 atm = 101.325 kPa = 760 mmHg), the molar volume of an ideal gas, Vm, and Vm,O2 is 22.414 and 22.392 L∙mol-1, respectively. Rounded to three decimal places, both values yield the conversion factor of 0.744 from units used in spiroergometry (VO2max [mL O2·min-1]) to SI units [µmol O2·s-1]. For comparison at normal temperature and pressure dry (NTPD: 20 °C), Vm,O2 is 24.038 L∙mol-1. Note that the SI standard pressure is 100 kPa, which corresponds to the standard molar volume of an ideal gas of 22.711 L∙mol-1 and 22.689 L∙mol-1 for O2. |
| SUIT | SUIT | SUIT is the abbreviation for Substrate-Uncoupler-Inhibitor Titration. SUIT protocols are used with mt-preparations to study respiratory control in a sequence of coupling and pathway control states induced by multiple titrations within a single experimental assay. These studies use biological samples economically to gain maximum information with a minimum amount of cells or tissue. |
| SUIT-001 | RP1 | 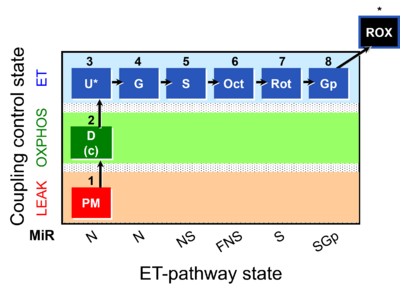 |
| SUIT-001 O2 ce-pce D003 | RP1 ce-pce | 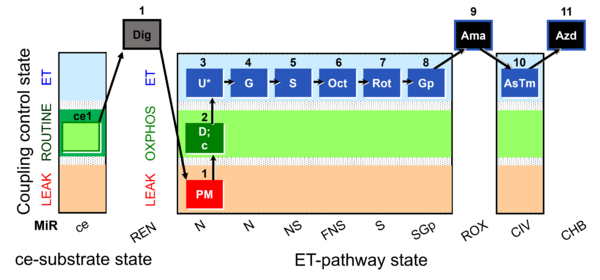 |
| SUIT-001 O2 ce-pce D004 | RP1 ce-pce blood | 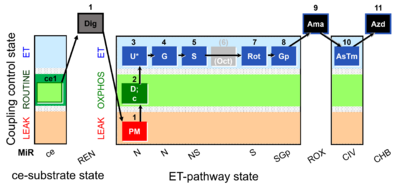 |
| SUIT-001 O2 mt D001 | RP1 mt | 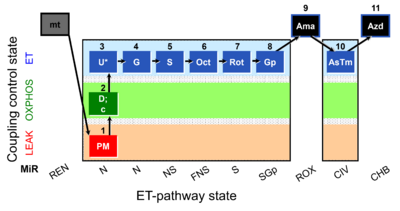 |
| SUIT-001 O2 pfi D002 | RP1 pfi | 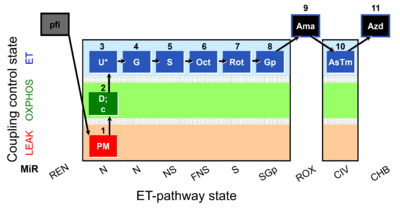 |
| SUIT-002 | RP2 | 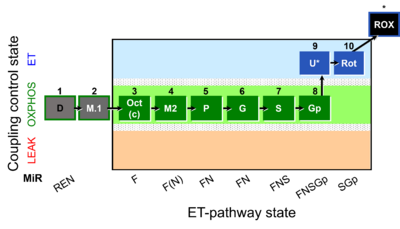 |
| SUIT-002 O2 ce-pce D007 | RP2 ce-pce | 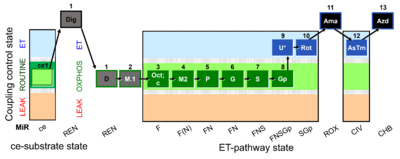 |
| SUIT-002 O2 ce-pce D007a | RP2 ce-pce blood |  |
| SUIT-002 O2 mt D005 | RP2 mt | 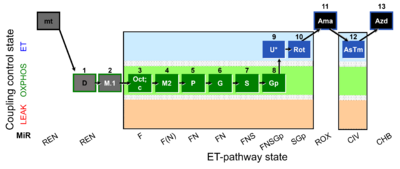 |
| SUIT-002 O2 pfi D006 | RP2 pfi | 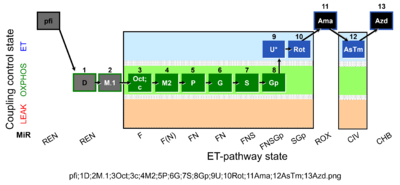 |
| SUIT-003 | CCP-ce | 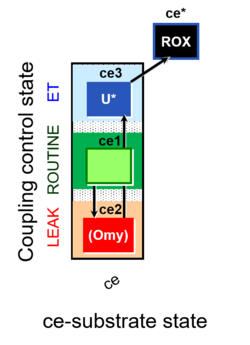 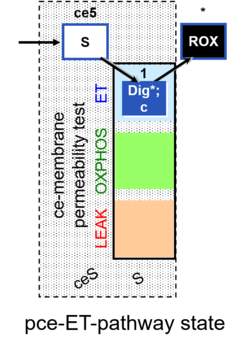 |
| SUIT-003 AmR ce D058 | AmR effect on ce | 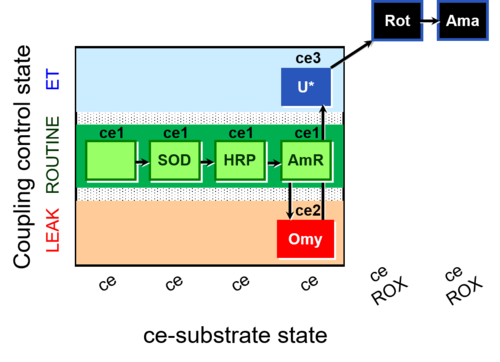 |
| SUIT-003 AmR ce D059 | AmR effect on ce - control | 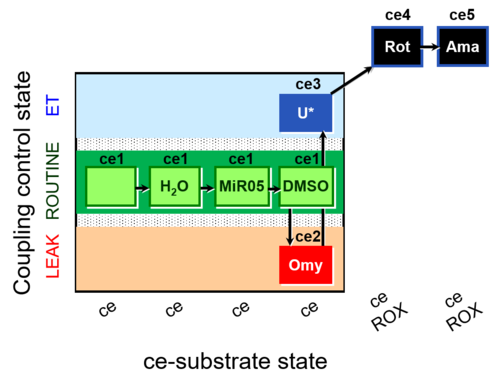 |
| SUIT-003 Ce1;ce1P;ce3U;ce4Glc;ce5M;ce6Rot;ce7S;1Dig;1c;2Ama;3AsTm;4Azd | cePMGlc,S |  |
| SUIT-003 Ce1;ce2U- | ce | 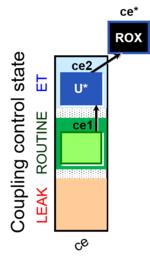 |
| SUIT-003 Ce1;ce3U- | ce | 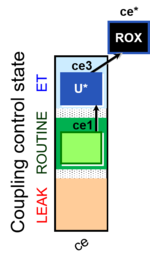 |
| SUIT-003 O2 ce D009 | CCP-ce short | 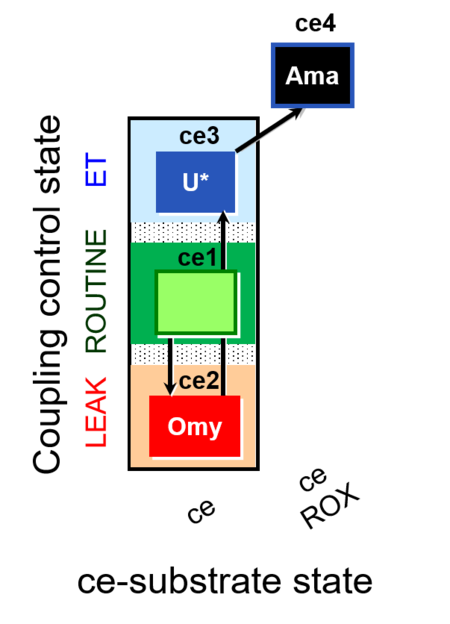 |
| SUIT-003 O2 ce D012 | CCP-ce(P) | 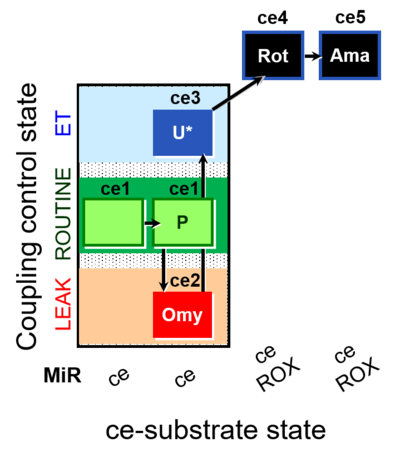 |
| SUIT-003 O2 ce D028 | CCP-ce S permeability test | 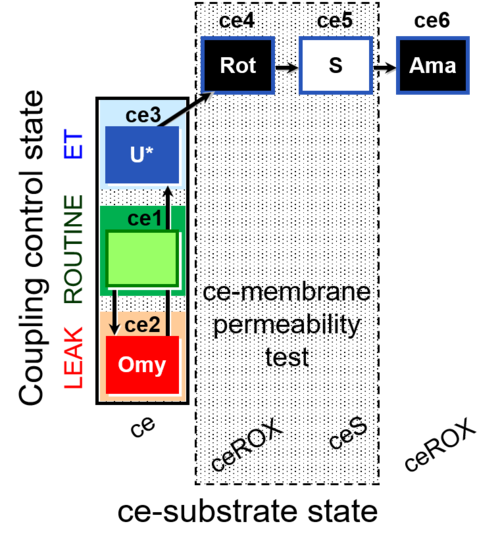 |
| SUIT-003 O2 ce D037 | CCP-ce Crabtree_R | 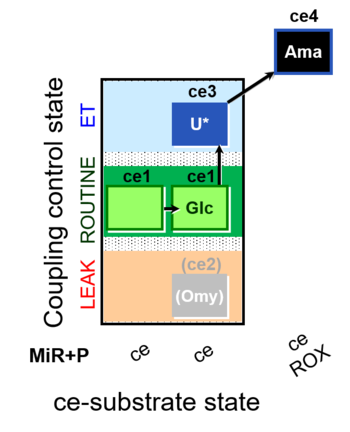 |
| SUIT-003 O2 ce D038 | CCP-ce Crabtree_E | 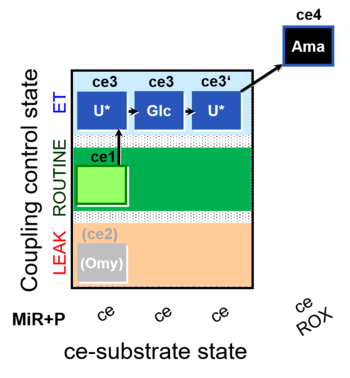 |
| SUIT-003 O2 ce D039 | CCP-ce microalgae | 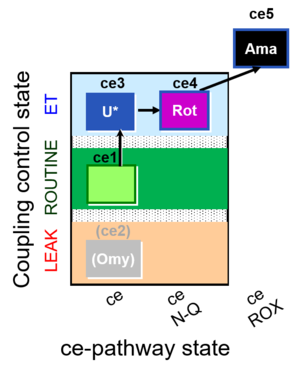 |
| SUIT-003 O2 ce D050 | CCP-ce Snv | 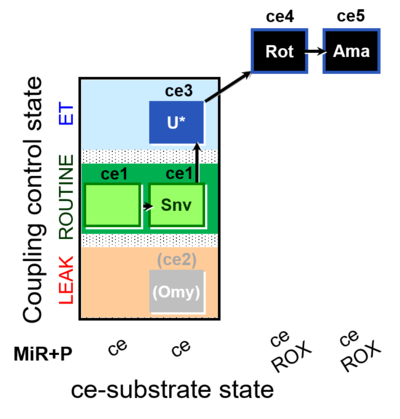 |
| SUIT-003 O2 ce D060 | CCP-ce Snv,Mnanv | 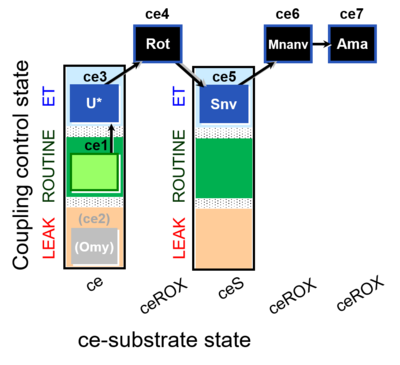 |
| SUIT-003 O2 ce D061 | CCP-ce Snv,Mnanv - control | 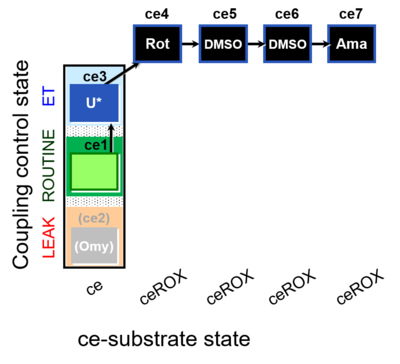 |
| SUIT-003 O2 ce D062 | CCP-ce Snv - control | 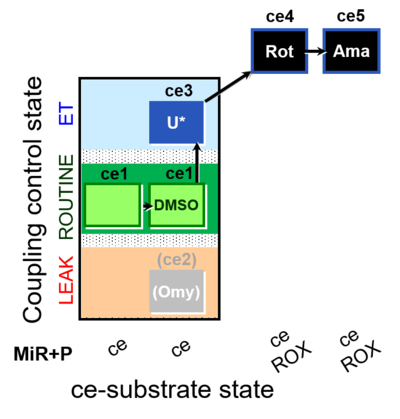 |
| SUIT-003 O2 ce-pce D013 | CCVP-Glc,M | 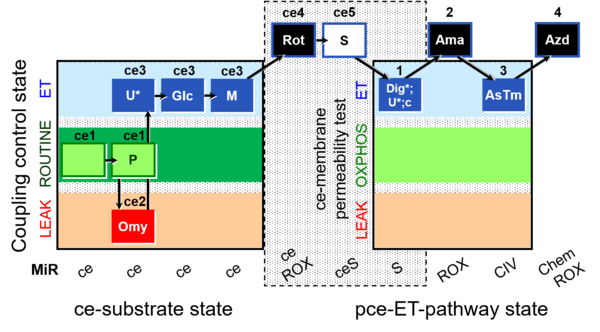 |
| SUIT-003 O2 ce-pce D018 | CCVP-Glc | 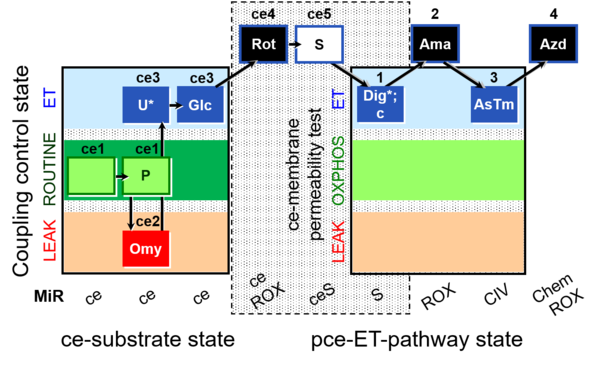 |
| SUIT-003 O2 ce-pce D020 | CCVP | 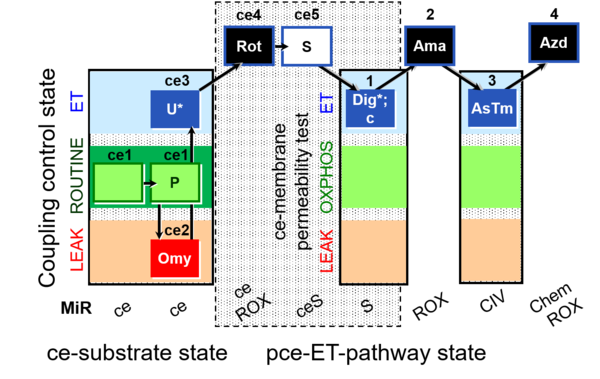 |
| SUIT-003 pH ce D067 | CCP-Crabtree with glycolysis inhibition | 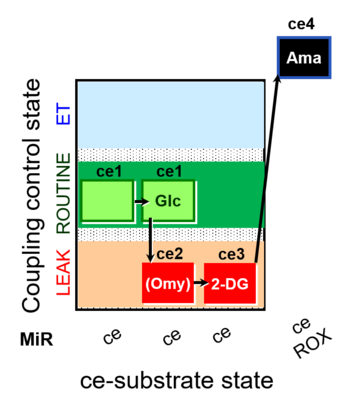 |
| SUIT-004 | RP1-short | 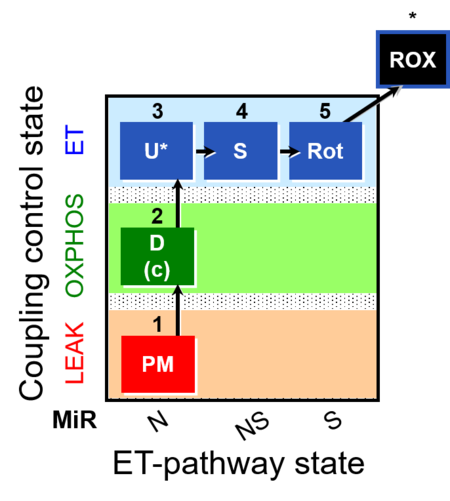 |
| SUIT-004 O2 pfi D010 | RP1-short pfi | 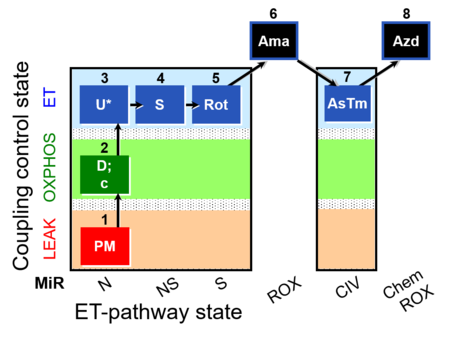 |
| SUIT-005 | RP2-short | 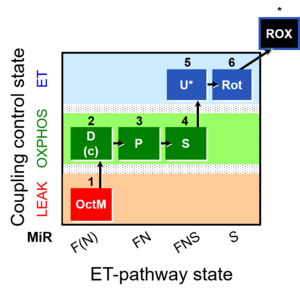 |
| SUIT-005 O2 pfi D011 | RP2-short pfi | 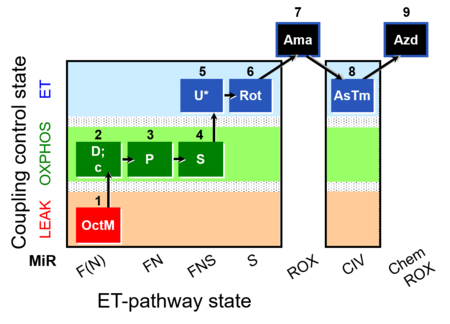 |
| SUIT-006 | CCP-mtprep |  |
| SUIT-006 O2 ce-pce D029 | CCP ce-pce PM | 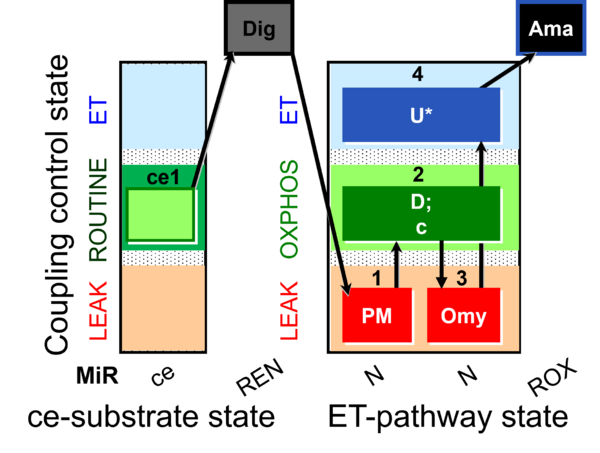 |
| SUIT-006 O2 mt D022 | CCP mt S(Rot) | 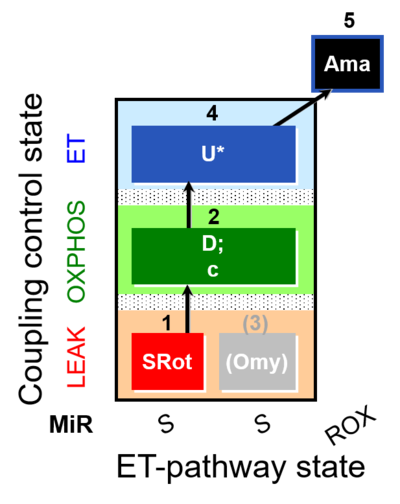 |
| SUIT-006 O2 mt D047 | CCP mt PM | 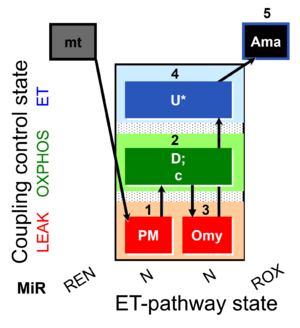 |
| SUIT-006 Q ce-pce D073 | CCP ce-pce S(Rot) | 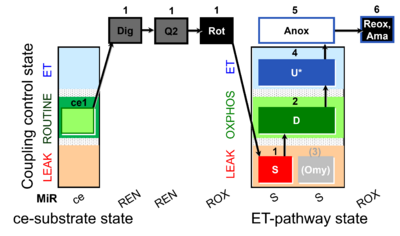 |
| SUIT-006 Q mt D071 | CCP mt S(Rot) | 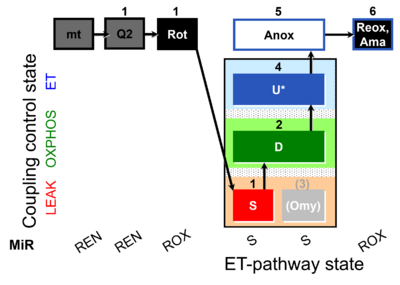 |
| SUIT-007 | Glutamate anaplerosis | 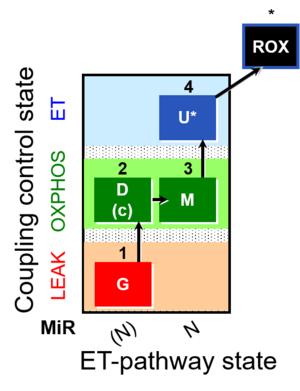 |
| SUIT-007 O2 ce-pce D030 | Glutamate anaplerotic pathway | 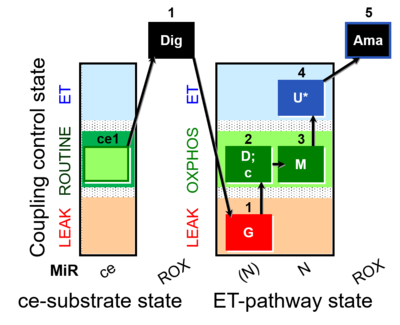 |
| SUIT-008 | PM+G+S_OXPHOS+Rot_ET | 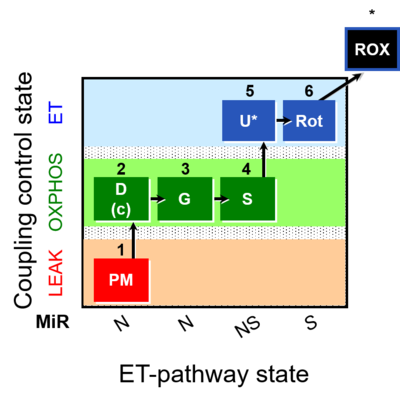 |
| SUIT-008 O2 ce-pce D025 | Q-junction ce-pce | 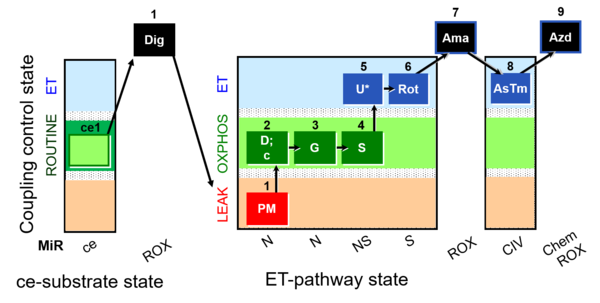 |
| SUIT-008 O2 mt D026 | Q-junction mtprep | 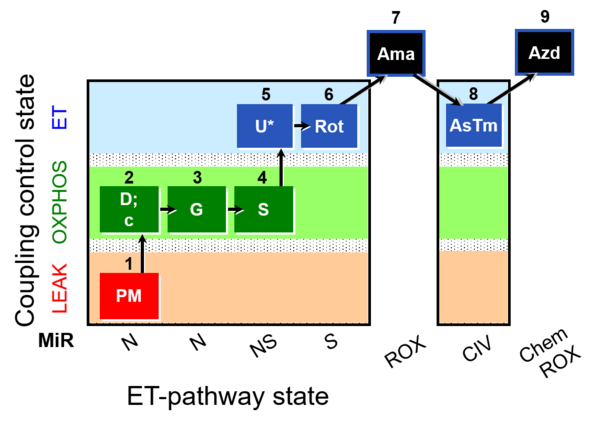 |
| SUIT-008 O2 pce D25 | NS(PGM) | 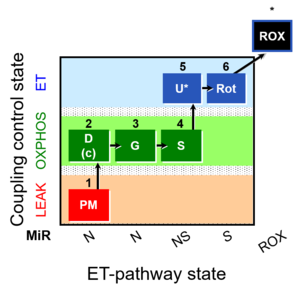 |
| SUIT-008 O2 pfi D014 | 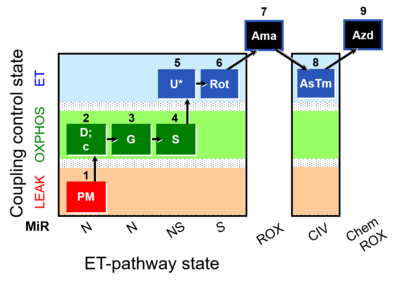 | |
| SUIT-009 O2 ce-pce D016 | 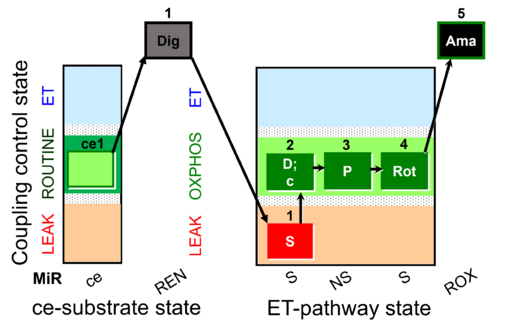 | |
| SUIT-009 O2 mt D015 | 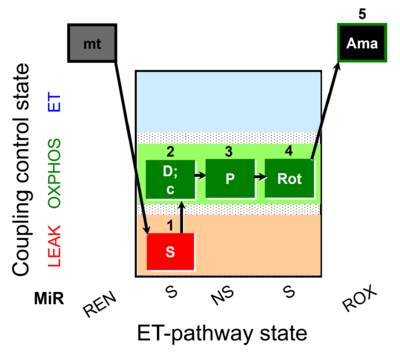 | |
| SUIT-010 | Digitonin test | 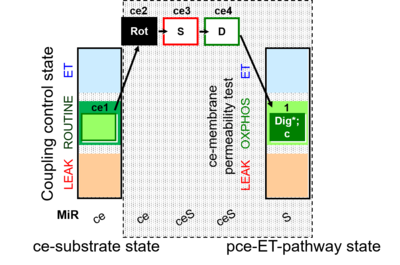 |
| SUIT-010 O2 ce-pce D008 | Dig titration-pce |  |
| SUIT-011 | GM+S_OXPHOS+Rot_ET | 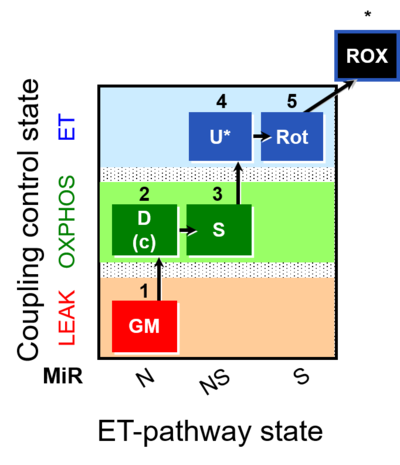 |
| SUIT-011 O2 pfi D024 | NS physiological maximum capapcity in fibres | 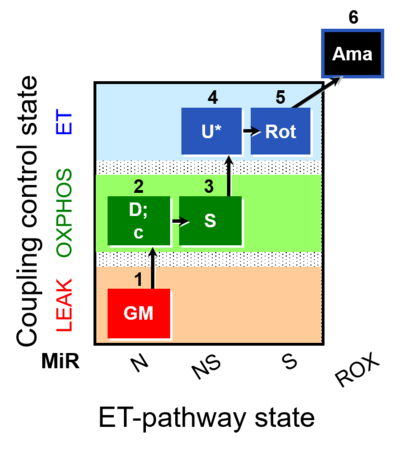 |
| SUIT-012 | PM+G_OXPHOS | 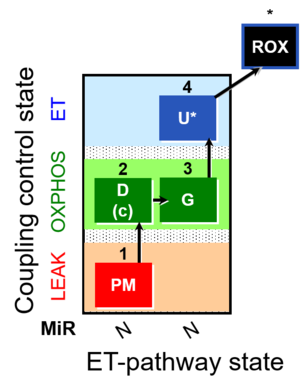 |
| SUIT-012 O2 ce-pce D052 | N(PGM) | 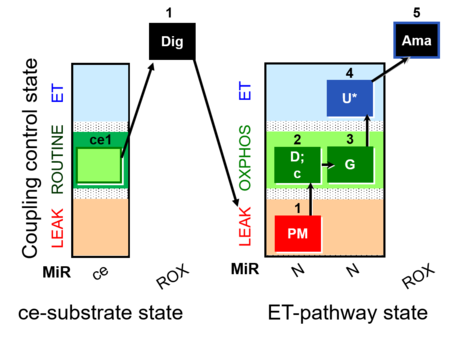 |
| SUIT-012 O2 mt D027 | N CCP mtprep | 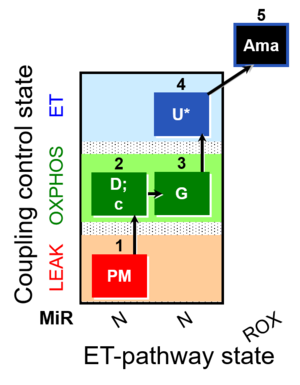 |
| SUIT-013 AmR ce D023 | O2 dependence of H2O2 production ce | 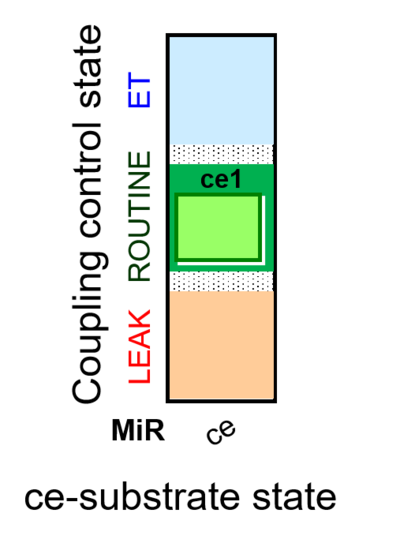 |
| SUIT-014 | GM+P+S_OXPHOS+Rot_ET | 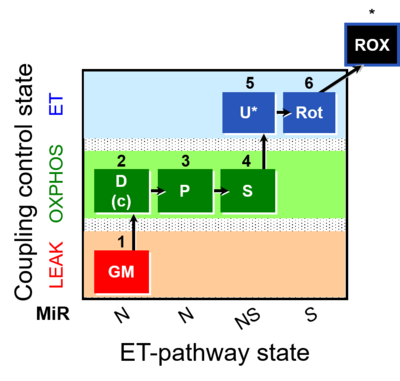 |
| SUIT-014 O2 pfi D042 | NS(PGM) | 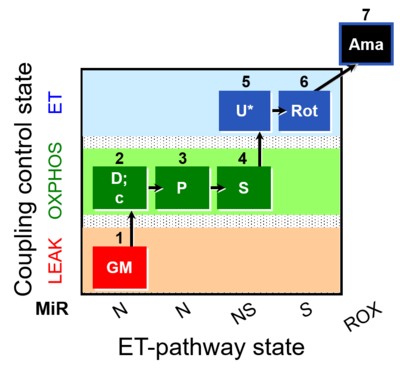 |
| SUIT-015 | F+G+P+S_OXPHOS+Rot_ET | 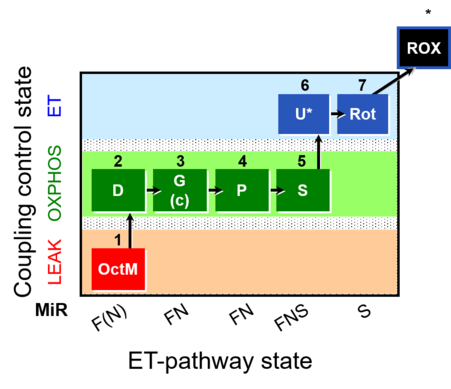 |
| SUIT-015 O2 pti D043 | FNS(Oct,PGM) | 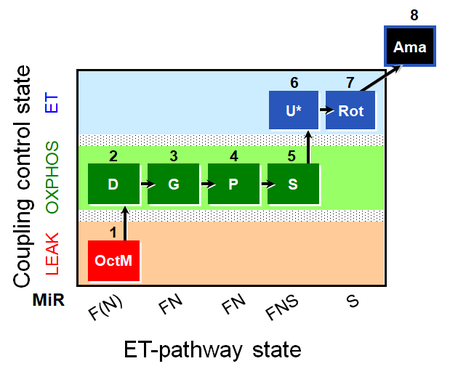 |
| SUIT-016 | F+G+S+Rot_OXPHOS+Omy | 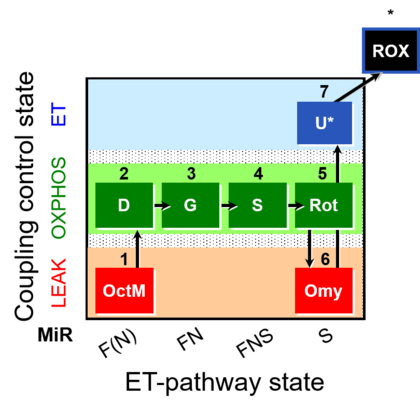 |
| SUIT-016 O2 pfi D044 | FNS(Oct,GM) | 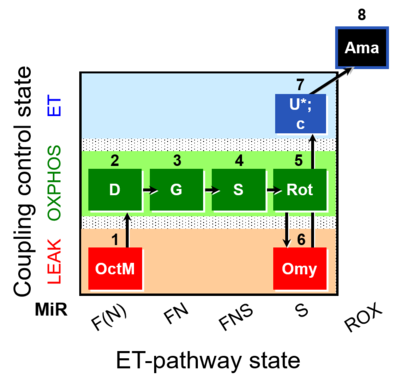 |
| SUIT-017 | F+G+S_OXPHOS+Rot_ET | 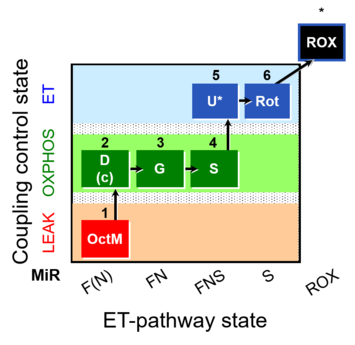 |
| SUIT-017 O2 mt D046 | FNS(Oct,GM) | 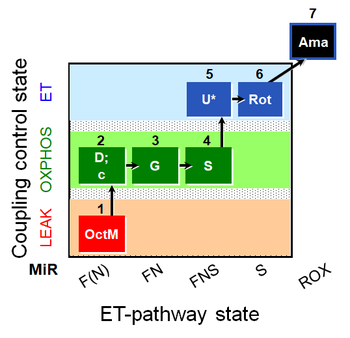 |
| SUIT-017 O2 pfi D049 | FNS(Oct,GM) |  |
| SUIT-018 O2 mt D054 |  | |
| SUIT-019 | Pal+Oct+P+G_OXPHOS+S+Rot_ET | 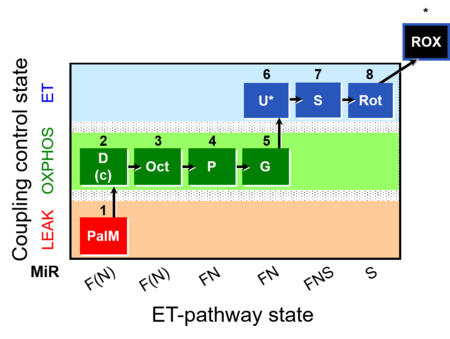 |
| SUIT-019 O2 pfi D045 | FNS(PalOct,PGM) | 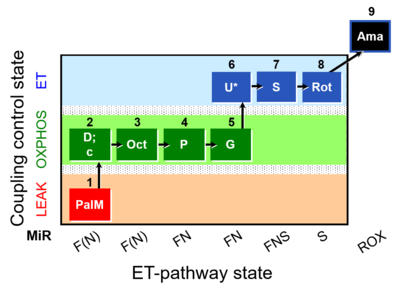 |
| SUIT-020 | PM+G+S+Rot_OXPHOS+Omy | 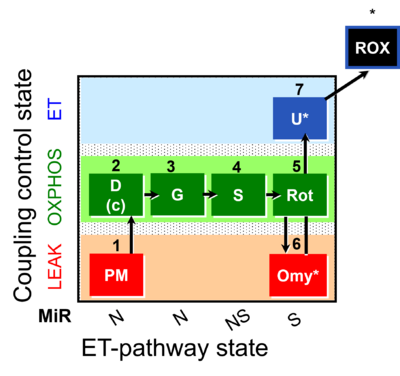 |
| SUIT-020 O2 mt D032 | Q-junction additivity and respiratory control for membrane potential | 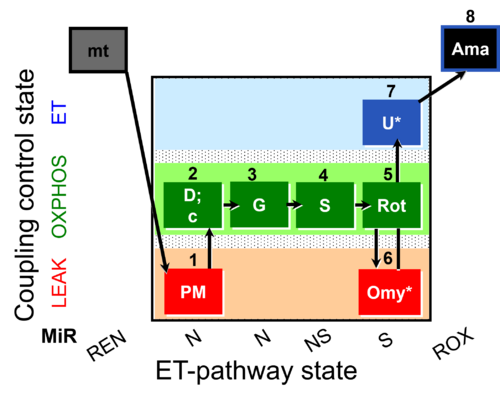 |
| SUIT-021 | OXPHOS (GM+S+Rot+Omy) | 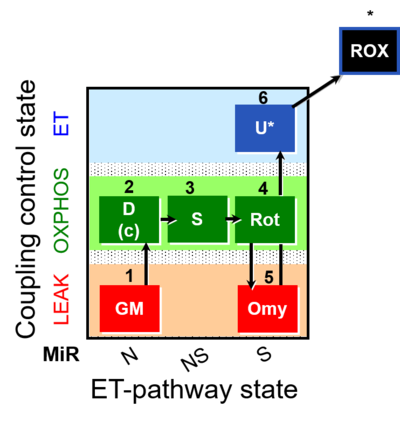 |
| SUIT-021 O2 mt D035 | NS(GM) | 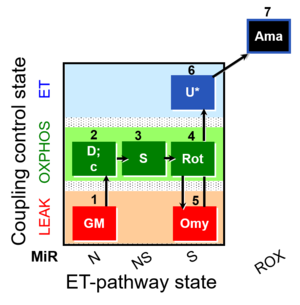 |
| SUIT-022 | AOX (ce CN+SHAM) | 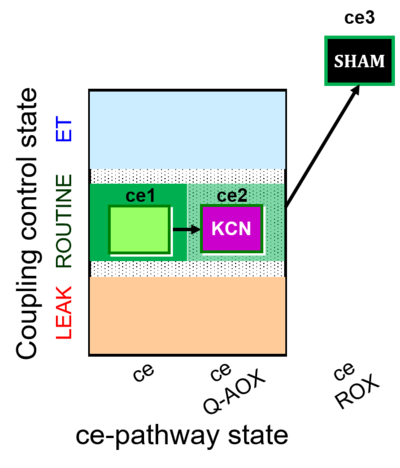 |
| SUIT-022 O2 ce D051 | AOX-ce CN+SHAM | 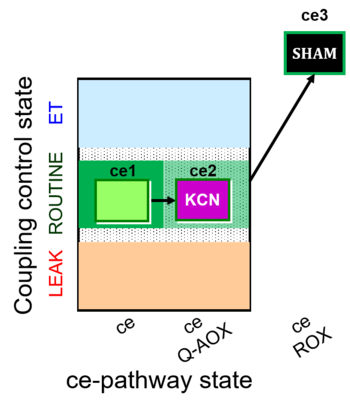 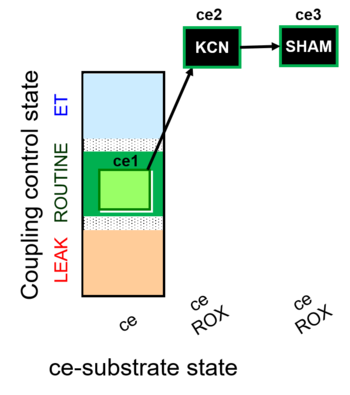 |
| SUIT-023 | AOX-ce SHAM+CN | 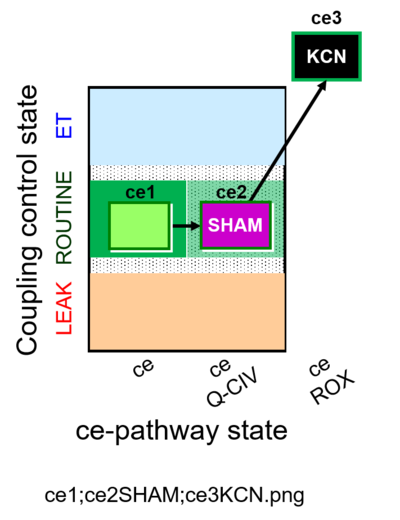 |
| SUIT-023 O2 ce D053 | AOX-ce SHAM+CN | 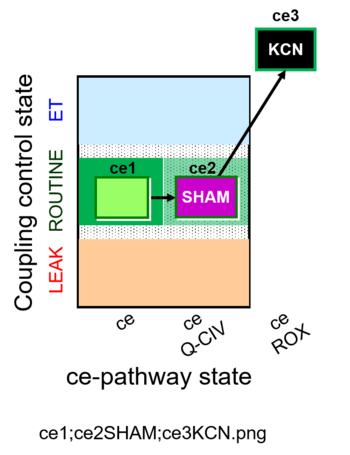 |
| SUIT-024 | ATPase (PM) | 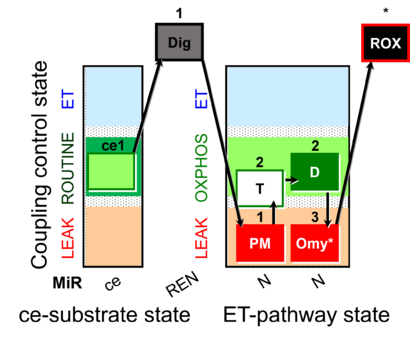 |
| SUIT-024 O2 ce-pce D056 | N(PM) | 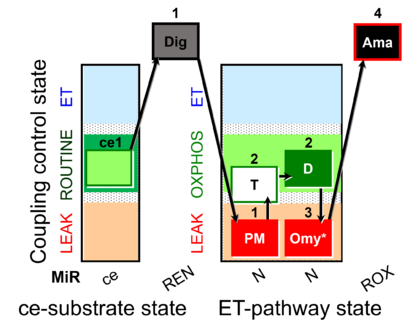 |
| SUIT-025 | OXPHOS (F+M+P+G+S+Rot) | 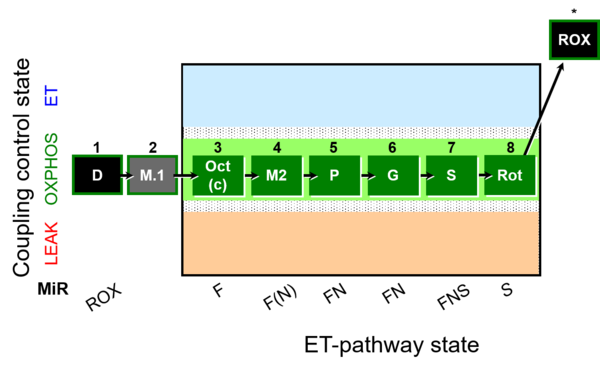 |
| SUIT-025 O2 mt D057 | FNS(Oct,PGM) | 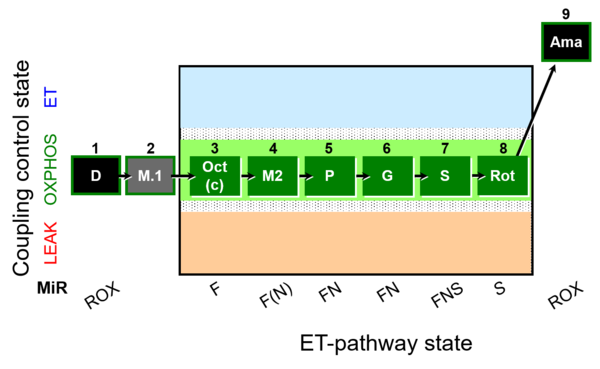 |
| SUIT-027 | Malate anaplerosis | 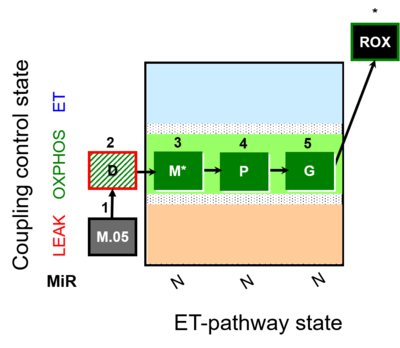 |
| SUIT-029 O2 mt D066 | QC_imt_PM_T+OXPHOS+c+Omy_ET_G+S+Rot | 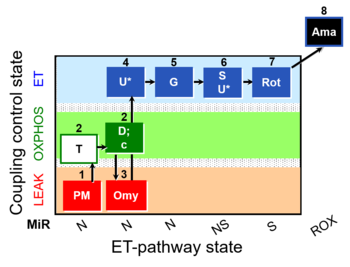 |
| SUIT-031 | PM+S+Rot | 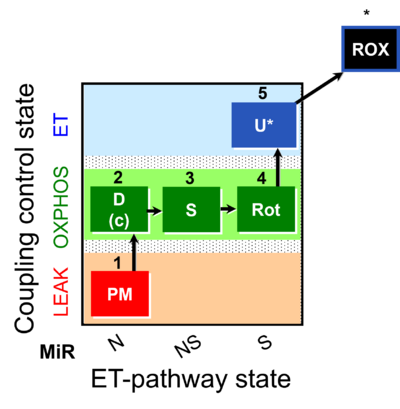 |
| SUIT-031 O2 ce-pce D079 | PM+S+Rot | 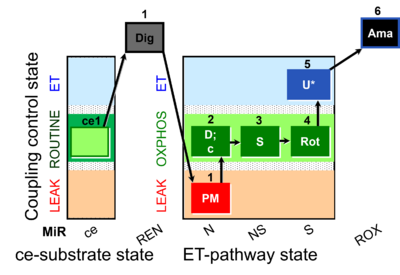 |
| SUIT-031 O2 mt D075 | PM+S+Rot |  |
| SUIT-031 Q ce-pce D074 | PM+S+Rot | 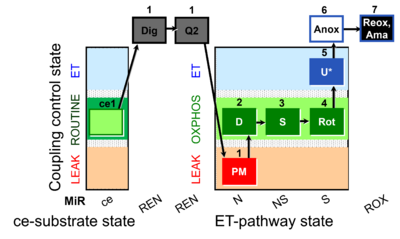 |
| SUIT-031 Q mt D072 | PM+S+Rot | 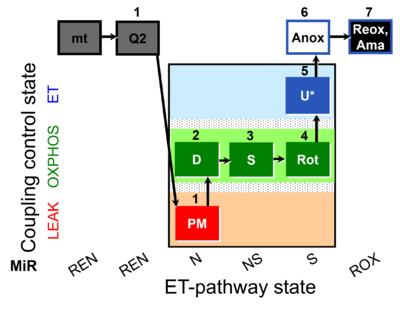 |
| SUITbrowser | Use the SUITbrowser to find the substrate-uncoupler-inhibitor-titration (SUIT) protocol most suitable for addressing your research questions. Open the SUITbrowser: http://suitbrowser.oroboros.at/ | |
| Select O2k - DatLab | Select O2k - DatLab | |
| Selectivity | Selectivity is the ability of a sensor or method to quantify accurately and specifically the analyte or analytes in the presence of other compounds. | |
| Sensitivity | Sensitivity refers to the response obtained for a given amount of analyte and is often denoted by two factors: the limit of detection and the limit of quantification. | |
| Smoothing | Various methods of smoothing can be applied to improve the signal-to-noise ratio. For instance, data points recorded over time [s] or over a range of wavelengths [nm] can be smoothed by averaging n data points per interval. Then the average of the n points per smoothing interval can be taken for each successively recorded data point across the time range or range of the spectrum to give a n-point moving average smoothing. This method decreases the noise of the signal, but clearly reduces the time or wavelength resolution. More advanced methods of smoothing are applied to retain a higher time resolution or wavelength resolution. | |
| Stability | Stability determines the accuracy of intensity and absorbance measurements as a function of time. Instability (see drift introduces systematic errors in the accuracy of fluorescence and absorbance measurements. | |
| Startup O2k-Respirometer | Startup O2k-Respirometer - the experimental system complete for basic high-resolution respirometry (HRR). The O2k-Respirometer includes the O2k-Main Unit with stainless steel housing, O2k-Assembly Kit, two OroboPOS (polarographic oxygen sensors) and OroboPOS-Service Kit, DatLab software, the ISS-Integrated Suction System, the O2k-Titration Set, and for performing high-resolution respirometry with reduced amounts of biological sample the O2k-sV-Module.
| |
| Steady state | A system is in a steady state if the state variables of a dynamic system do not change over time due to exchange processes with the environment, which compensate for internal dissipative transformations — such as chemical reactions or diffusion — and thus prevent any changes of the system and externalize dissipative changes to the environment. The dynamic nature of the steady state differentiates it from the thermodynamic equilibrium state. {Quote} Steady states can be obtained only in open systems, in which changes by internal transformations, e.g., O2 consumption, are instantaneously compensated for by external fluxes across the system boundary, e.g., O2 supply, thus preventing a change of O2 concentration in the system (Gnaiger 1993). Mitochondrial respiratory states monitored in closed systems satisfy the criteria of pseudo-steady states for limited periods of time, when changes in the system (concentrations of O2, fuel substrates, ADP, Pi, H+) do not exert significant effects on metabolic fluxes (respiration, phosphorylation). Such pseudo-steady states require respiratory media with sufficient buffering capacity and substrates maintained at kinetically-saturating concentrations, and thus depend on the kinetics of the processes under investigation. {end of Quote: BEC 2020.1}. Whereas fluxes may change at a steady state over time, concentrations are maintained constant. The 'respiratory steady state' (Chance and Williams 1955) is characterized by constant fluxes (O2 flux, H2O2 flux) and measured variables of state (cytochrome redox states, Q redox state, NADH redox state, mitochondrial membrane potential). High-resolution respirometry allows for the measurement of several parameters (e.g. O2 flux, H2O2 flux, mitochondrial membrane potential) at pseudo-steady states, when changes of concentrations in the closed system do not exert any control on fluxes. Combination with the Titration-Injection microPump (TIP2k) allows operation with programmable titration regimes at steady-state ADP concentration (Gnaiger 2001), oxygen concentration (oxystat mode; Gnaiger et al 2000, Harrison et al 2015) or steady-state pH (pH-stat more), yielding an expanded flexibility in experimental design by combining the technical advantages of closed and open systems approaches. | |
| Substrate control state | See Electron-transfer-pathway state | |
| Substrate-uncoupler-inhibitor titration | SUIT | Mitochondrial Substrate-uncoupler-inhibitor titration (SUIT) protocols are used with mitochondrial preparations to study respiratory control in a sequence of coupling and substrates states induced by multiple titrations within a single experimental assay. |
| TIP2k-Module | TIP2k-Module - Titration-Injection microPump (TIP2k) for two-channel operation with the O2k-FluoRespirometer with automatic control by DatLab of programmable titration regimes and feedback control (oxystat, pH-stat). | |
| TPP+ inhibitory effect | A major task in establishing a procedure for measurement of mitochondrial membrane potential using probe molecules is the evaluation of inhibitory concentrations of the probe molecule on the activity of respiration. The TPP+ inhibitory effect (this also applies to TPMP+ and other indicator molecules) is frequently ignored. Accurate knowledge of a threshold concentration is required to evaluate the necessary limit of detection of TPP+, and for restriction of experimental TPP+ concentrations below the inhibitory range. | |
| Taurine | Taurine, or 2-Aminoethan sulfonic acid, is one of the most abundant low-molecular-weight organic constituents in animals and humans. It has a multitude of functions in different types of tissue, one of which is the stabilization of membranes. Because of this and its antioxidative effect, taurine is a component of the respiration media MiR05 and MiR06 to preserve mitochondrial function. | |
| Tetraphenylphosphonium | TPP+ | Tetraphenylphosphonium (TPP+). A lipophilic molecular probe in conjunction with an ion selective electrode (ISE) for measuring the mitochondrial membrane potential. |
| Time resolution | Time resolution in respirometric measurements is influenced by three parameters: the response time of the POS, the data sampling interval and the number of points used for flux calculation. | |
| Uncoupler titrations | In uncoupler titrations various uncouplers, such as CCCP, FCCP or DNP are applied to uncouple mitochondrial electron transfer from phosphorylation (ATP synthase, ANT and phosphate carrier), particularly with the aim to measure ET capacity. ET capacity is maximum oxygen flux measured as noncoupled respiration with optimum uncoupler concentration. | |
| Uncoupling-control ratio | UCR | The uncoupling-control ratio UCR is the ratio of ET-pathway/ROUTINE-respiration (E/R) in living cells, evaluated by careful uncoupler titrations (Steinlechner et al 1996). Compare ROUTINE-control ratio (R/E) (Gnaiger 2008). |
| Unspecific binding of TPP+ | Unspecific binding of the probe molecule TPP+ in the matrix phase of mitochondria is taken into account as a correction for measurement of the mitochondrial membrane potential. External unspecific binding is the binding outside of the inner mt-membrane or on the outer side of the inner mt-membrane, in contrast to internal unspecific binding. | |
| VO2max | VO2max; VO2max/M | Maximum oxygen consumption, VO2max, is and index of cardiorespiratory fitness, measured by spiroergometry on human and animal organisms capable of controlled physical exercise performance on a treadmill or cycle ergometer. VO2max is the maximum respiration of an organism, expressed as the volume of O2 at STPD consumed per unit of time per individual object [mL.min-1.x-1]. If normalized per body mass of the individual object, M [kg.x-1], mass specific maximum oxygen consumption, VO2max/M, is expressed in units [mL.min-1.kg-1]. |
| Warburg effect | Recently, controversies had a renaissance on the much neglected Crabtree effect (aerobic glycolysis in a large range of cells exposed to glucose or fructose, with fully functional mitochondria; Crabtree 1929; Gnaiger and Kemp 1990) versus the Warburg effect (loss of mitochondrial function inducing cancer and stimulating compensatory aerobic glycolysis in the presence of oxygen; Warburg 1956; see list of references for reviews). Today it is widely accepted that ‘the Warburg effect is not consistent across all cancer types’ (Potter et al 2016) and reprogramming of mitochondrial energy metabolism represents a functional adjustment of cancer cells (Schöpf et al 2020). | |
| Zero calibration | R0 | Zero calibration is, together with air calibration, one of the two steps of the POS calibration. It is performed in the closed chamber after all the oxygen has been depleted by the addition of dithionite or by respiration of imt or cells. Any incubation medium can be used for zero calibration with dithionite or sample. Unlike air calibration, it is not necessary to perform a zero calibration on each experimental day. After performing a zero calibration, it is recommended not running other experiments on the same day. Even after standard cleaning of the O2k-chambers, there might be residual amounts of reduced dithionite in the chamber, affecting the oxygen flux in subsequent experiments performed on the same day. |