Superoxide: Difference between revisions
From Bioblast
(Created page with "{{MitoPedia |abbr=O<sub>2</sub><sup>-•</sup> |description=left|60px|Superoxide anion '''Superoxide anion''', O<sub>2</sub><sup>-•</sup>, is a free radical fo...") |
No edit summary |
||
| (2 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
{{MitoPedia | {{MitoPedia | ||
|abbr=O<sub>2</sub><sup>- | |abbr=O<sub>2</sub><sup>•-</sup> | ||
|description=[[File:O2-.jpg|left|60px|Superoxide anion]] | |description=[[File:O2-.jpg|left|60px|Superoxide anion]] | ||
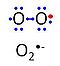
'''Superoxide anion''', O<sub>2</sub><sup>- | '''Superoxide anion''', O<sub>2</sub><sup>•-</sup>, is a free radical formed in a one-electron reduction of molecular [[oxygen]] (red bullet in the figure), yielding a negatively charged molecule with a single unpaired electron (blue bullet on the left). It is highly reactive with organic compounds, and its intracellular concentration is kept under control by [[superoxide dismutase]]. | ||
|info=[[Fridovich_1997_J Biol Chem]] | |||
}} | }} | ||
{{MitoPedia methods}} | {{MitoPedia methods}} | ||
Latest revision as of 03:57, 16 April 2014
Description
Superoxide anion, O2•-, is a free radical formed in a one-electron reduction of molecular oxygen (red bullet in the figure), yielding a negatively charged molecule with a single unpaired electron (blue bullet on the left). It is highly reactive with organic compounds, and its intracellular concentration is kept under control by superoxide dismutase.
Abbreviation: O2•-
Reference: Fridovich_1997_J Biol Chem
MitoPedia topics: Substrate and metabolite