Hasan 2012 Phys Chem Chem Phys
| Hasan SS, Cramer WA (2012) On rate limitations of electron transfer in the photosynthetic cytochrome b6f complex. Phys Chem Chem Phys 14:13853-60. https://doi.org/10.1039/c2cp41386h |
Hasan SS, Cramer WA (2012) Phys Chem Chem Phys
Abstract: Considering information in the crystal structures of the cytochrome b(6)f complex relevant to the rate-limiting step in oxygenic photosynthesis, it is enigmatic that electron transport in the complex is not limited by the large distance, approximately 26 Å, between the iron-sulfur cluster (ISP) and its electron acceptor, cytochrome f. This enigma has been explained for the respiratory bc(1) complex by a crystal structure with a greatly shortened cluster-heme c(1) distance, leading to a concept of ISP dynamics in which the ISP soluble domain undergoes a translation-rotation conformation change and oscillates between positions relatively close to the cyt c(1) heme and a membrane-proximal position close to the ubiquinol electron-proton donor. Comparison of cytochrome b(6)f structures shows a variation in cytochrome f heme position that suggests the possibility of flexibility and motion of the extended cytochrome f structure that could entail a transient decrease in cluster-heme f distance. The dependence of cyt f turnover on lumen viscosity is consistent with a role of ISP - cyt f dynamics in determination of the rate-limiting step under conditions of low light intensity. Under conditions of low light intensity and proton electrochemical gradient present, for example, under a leaf canopy, it is proposed that a rate limitation of electron transport in the b(6)f complex may also arise from steric constraints in the entry/exit portal for passage of the plastoquinol and -quinone to/from its oxidation site proximal to the iron-sulfur cluster.
• Bioblast editor: Gnaiger E
Hydrogen ion ambiguities in the electron transfer system
Communicated by Gnaiger E (2023-10-08) last update 2023-11-10
- Electron (e-) transfer linked to hydrogen ion (hydron; H+) transfer is a fundamental concept in the field of bioenergetics, critical for understanding redox-coupled energy transformations.
- However, the current literature contains inconsistencies regarding H+ formation on the negative side of bioenergetic membranes, such as the matrix side of the mitochondrial inner membrane, when NADH is oxidized during oxidative phosphorylation (OXPHOS). Ambiguities arise when examining the oxidation of NADH by respiratory Complex I or succinate by Complex II.
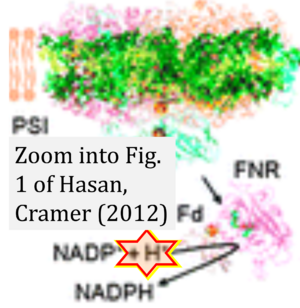
- Oxidation of NADH or succinate involves a two-electron transfer of 2{H++e-} to FMN or FAD, respectively. Figures indicating a single electron e- transferred from NADH or succinate lack accuracy.
- The oxidized NAD+ is distinguished from NAD indicating nicotinamide adenine dinucleotide independent of oxidation state.
- NADH + H+ → NAD+ +2{H++e-} is the oxidation half-reaction in this H+-linked electron transfer represented as 2{H++e-} (Gnaiger 2023). Putative H+ formation shown as NADH → NAD+ + H+ conflicts with chemiosmotic coupling stoichiometries between H+ translocation across the coupling membrane and electron transfer to oxygen. Ensuring clarity in this complex field is imperative to tackle the apparent ambiguity crisis and prevent confusion, particularly in light of the increasing number of interdisciplinary publications on bioenergetics concerning diagnostic and clinical applications of OXPHOS analysis.