Difference between revisions of "ET capacity"
| Line 6: | Line 6: | ||
|type=Respiration | |type=Respiration | ||
}} | }} | ||
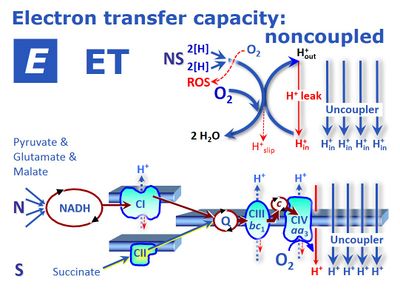
[[File:ETS-NS.jpg|400px|thumb|Noncoupled respiration with a shortcircuit of the proton cycle across the inner mt-membrane at optimum uncoupler (protonophore) concentration stimulating maximum oxygen flux. 2[H] indicates the reduced hydrogen equivalents of CHO substrates and electron transfer to oxygen. H<sup>+</sup><sub>out</sub> are protons pumped out of the matrix phase. Proton leaks dissipate energy of translocated protons. ETS capacity is not limited by the capacity of the phosphorylation system (uncontrolled state). Measurement of ETS capacity is possible by uncoupler titrations in intact cells and in mt-preparations supported by an ETS-competent substrate state, exemplifed as the NS-pathway (CI<small>&</small>II-linked substrate supply). Modified after [[Gnaiger 2014 MitoPathways]]).]] | |||
{{MitoPedia concepts | {{MitoPedia concepts | ||
|mitopedia concept=Respiratory state | |mitopedia concept=Respiratory state | ||
| Line 20: | Line 21: | ||
__TOC__ | __TOC__ | ||
= Why ETS, why not State 3u? = | = Why ETS, why not State 3u? = | ||
{{Publication | {{Publication | ||
|title=Gnaiger E (2014) Why ETS, why not State 3u? Mitochondr Physiol Network 2014-07-06. | |title=Gnaiger E (2014) Why ETS, why not State 3u? Mitochondr Physiol Network 2014-07-06. | ||
| Line 37: | Line 37: | ||
[[File:Hatefi 1962 NS 2012.jpg|right|500px|Q-junction]] | [[File:Hatefi 1962 NS 2012.jpg|right|500px|Q-junction]] | ||
== Electron transfer system versus electron transport chain == | == Electron transfer system versus electron transport chain == | ||
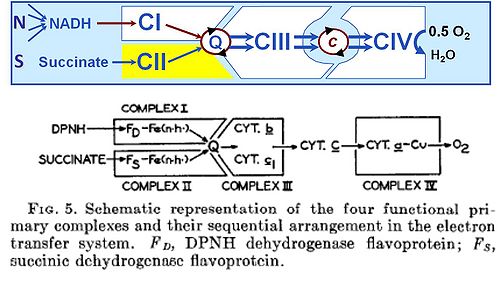
The well established terms 'respiratory chain' or 'electron transfer chain' suggest erroneously that the convergent '''electron transfer system''' may be designed as a simple ''chain''. But the term '''electron transport chain''' (or electron transfer chain, ETC) is a misnomer. Understanding mitochondrial respiratory control has suffered greatly from this inappropriate terminology, although textbooks using the term ETC (Lehninger 1970) make it sufficiently clear that '''electron transfer systems are not arranged as a chain''': the „ETC‟ is in fact not a simple chain but an arrangement of electron transfer complexes in a non-linear, convergent electron transfer system. The classically introduced term '''electron transfer system''' (Hatefi et al 1962 | :::: The well established terms 'respiratory chain' or 'electron transfer chain' suggest erroneously that the convergent '''electron transfer system''' may be designed as a simple ''chain''. But the term '''electron transport chain''' (or electron transfer chain, ETC) is a misnomer. Understanding mitochondrial respiratory control has suffered greatly from this inappropriate terminology, although textbooks using the term ETC (Lehninger 1970) make it sufficiently clear that '''electron transfer systems are not arranged as a chain''': the „ETC‟ is in fact not a simple chain but an arrangement of electron transfer complexes in a non-linear, convergent electron transfer system. The classically introduced term '''electron transfer system''' (Hatefi et al 1962) is accurate and sufficient (IUB 1991). | ||
The established convention of defining the 'electron transport chain' as being comprised of four Complexes has conceptual weaknesses. | :::: The established convention of defining the 'electron transport chain' as being comprised of four Complexes has conceptual weaknesses. | ||
(a) In fact, there are at least six Complexes of mitochondrial electron transfer: In addition to Complexes I and II, [[glycerophosphate dehydrogenase complex]] (CGpDH) and [[electron-transferring flavoprotein complex]] (CETF) are involved in the [[Q-junction]] with electron transfer to [[Complex III]] | :::: (a) In fact, there are at least six Complexes of mitochondrial electron transfer: In addition to Complexes I and II, [[glycerophosphate dehydrogenase complex]] (CGpDH) and [[electron-transferring flavoprotein complex]] (CETF) are involved in the [[Q-junction]] with electron transfer to [[Complex III]] (IUB 1991, Gnaiger 2014). | ||
(b) The term „chain‟ suggests a linear sequence, whereas the functional structure of the electron transfer system can only be understood by recognizing '''the convergence of electron flow at the Q-junction''', followed by a chain of Complexes III and IV, mediated by [[cytochrome c | cytochrome ''c'']] | :::: (b) The term „chain‟ suggests a linear sequence, whereas the functional structure of the electron transfer system can only be understood by recognizing '''the convergence of electron flow at the Q-junction''', followed by a chain of Complexes III and IV, mediated by [[cytochrome c | cytochrome ''c'']] (Gnaiger 2014). | ||
Electrons flow to oxygen from either [[Complex I]] with a total of three coupling sites, or from [[Complex II]] and other flavoproteins, providing multiple entries into the Q-cycle with two coupling sites downstream | :::: Electrons flow to oxygen from either [[Complex I]] with a total of three coupling sites, or from [[Complex II]] and other flavoproteins, providing multiple entries into the Q-cycle with two coupling sites downstream (Gnaiger 2014). | ||
== Electron transfer versus transport == | == Electron transfer versus transport == | ||
Electron transfer and electron transport are used synonymously. A general distinction, however, may be helpful: | :::: Electron transfer and electron transport are used synonymously. A general distinction, however, may be helpful: | ||
(i) '''Transfer''' (inter- or intramolecular) of a reactant involves a chemical reaction. | :::: (i) '''Transfer''' (inter- or intramolecular) of a reactant involves a chemical reaction. | ||
(ii) '''Transport''' (from one place to another) of an entity is a (vectorial) process in contrast to a chemical reaction | :::: (ii) '''Transport''' (from one place to another) of an entity is a (vectorial) process in contrast to a chemical reaction (IUPAC Green Book). | ||
== The important difference between states ''P'' and ''E'' == | == The important difference between states ''P'' and ''E'' == | ||
The abbreviation '''[[State 3u]]''' is used frequently in bioenergetics, to indicate the noncoupled state of maximum respiration, ''E'',<ref> Gnaiger E. Electron transfer system versus electron transport chain. Mitochondr Physiol Network. »[[Electron transfer system]]«</ref> without sufficient emphasis on the fundamental difference between state ''P'' ([[OXPHOS capacity]]; coupled, with an uncoupled component; State 3) and state ''E'' ([[ETS capacity]], noncoupled) | :::: The abbreviation '''[[State 3u]]''' is used frequently in bioenergetics, to indicate the noncoupled state of maximum respiration, ''E'',<ref> Gnaiger E. Electron transfer system versus electron transport chain. Mitochondr Physiol Network. »[[Electron transfer system]]«</ref> without sufficient emphasis on the fundamental difference between state ''P'' ([[OXPHOS capacity]]; coupled, with an uncoupled component; State 3) and state ''E'' ([[ETS capacity]], noncoupled) (Gnaiger 2009, 2014). | ||
::::* '''''P''=''E''''': The specific case of equal OXPHOS and ETS capacity (''P/E''=1) yields the important information that the capacity of the [[phosphorylation system]] matches or is in potential excess of the ETS capacity, such that OXPHOS capacity is not limited by the phosphorylation system in the specific mitochondria. This varies with species and tissues, and changes as a result of pathologies due to defects in the phosphorylation system. An example for ''P/E''=1 is mouse skeletal muscle mitochondria (Aragones et al 2008). | |||
* '''''P'' | ::::* '''''P''<''E''''': When OXPHOS is less than ETS capacity, the phosphorylation system limits OXPHOS capacity, and there is an apparent ETS excess capacity. For example, this is the case in healthy human skeletal muscle mitochondria (Pesta et al 2011). | ||
* '''''P'' | ::::* '''''P''>''E''''': If ETS is less than OXPHOS capacity in intact cells, or in mitochondrial preparations with defined substrate(s), then you have encountered an experimental artefact, and the apparent ETS capacity is too low. Artificially low ETS capacity may be obtained due to overtitration of [[uncoupler]]. Inhibitors of ATP synthase may suppress ETS capacity in intact cells, particularly in stressed cells. | ||
== Consequences for evaluation of coupling == | == Consequences for evaluation of coupling == | ||
In some textbooks on Bioenergetics, the [[RCR]] is defined as either the State 3/State 4 ratio or the State 3u/State 4 ratio. This reflects lack of conceptual distinction between State 3 (or ''P'') and 3u (''E''), and clarification is best achieved by avoiding ambiguous terminology. [[RCR]] as defined originally is the 'acceptor control ratio' or 'adenylate control ratio' (see [[LEAK control ratio]], ''L/E'' | :::: In some textbooks on Bioenergetics, the [[RCR]] is defined as either the State 3/State 4 ratio or the State 3u/State 4 ratio. This reflects lack of conceptual distinction between State 3 (or ''P'') and 3u (''E''), and clarification is best achieved by avoiding ambiguous terminology. [[RCR]] as defined originally is the 'acceptor control ratio' or 'adenylate control ratio' (see [[LEAK control ratio]], ''L/E''; [[biochemical coupling efficiency]]). ETS capacity but not OXPHOS capacity provides a valid reference for an index of uncoupling. | ||
== Related terms in Bioblast == | == Related terms in Bioblast == | ||
[[Image:OXPHOS-coupled energy cycles.jpg|right|300px||link=Gnaiger 2014 MitoPathways|OXPHOS-coupled energy cycles. Source: The blue book]] | [[Image:OXPHOS-coupled energy cycles.jpg|right|300px||link=Gnaiger 2014 MitoPathways|OXPHOS-coupled energy cycles. Source: The blue book]] | ||
[[File:P.jpg |link=OXPHOS capacity]] [[OXPHOS capacity |OXPHOS]], ''P'' | :::: [[File:P.jpg |link=OXPHOS capacity]] [[OXPHOS capacity |OXPHOS]], ''P'' | ||
[[File:R.jpg |link=ROUTINE respiration]] [[ROUTINE respiration |ROUTINE]], ''R'' | :::: [[File:R.jpg |link=ROUTINE respiration]] [[ROUTINE respiration |ROUTINE]], ''R'' | ||
[[File:E.jpg |link=ETS capacity]] [[ETS capacity |ETS]], ''E'' | :::: [[File:E.jpg |link=ETS capacity]] [[ETS capacity |ETS]], ''E'' | ||
[[File:L.jpg |link=LEAK respiration]] [[LEAK respiration |LEAK]], ''L'' | :::: [[File:L.jpg |link=LEAK respiration]] [[LEAK respiration |LEAK]], ''L'' | ||
[[File:ROX.jpg |link=Residual oxygen consumption]] [[Residual oxygen consumption |ROX]], ''R'' | :::: [[File:ROX.jpg |link=Residual oxygen consumption]] [[Residual oxygen consumption |ROX]], ''R'' | ||
=== The ETS state === | === The ETS state === | ||
* [[ETS-competent substrate state]] | ::::* [[ETS-competent substrate state]] | ||
* [[Level flow]] | ::::* [[Level flow]] | ||
* [[Noncoupled respiration]] - [[Uncoupler]]<ref>Gnaiger E. Is respiration uncoupled - noncoupled - dyscoupled? Mitochondr Physiol Network. »[[Uncoupler]]«</ref> | ::::* [[Noncoupled respiration]] - [[Uncoupler]]<ref>Gnaiger E. Is respiration uncoupled - noncoupled - dyscoupled? Mitochondr Physiol Network. »[[Uncoupler]]«</ref> | ||
* [[Coupling control protocol]] | ::::* [[Coupling control protocol]] | ||
=== ETS-related flux control factors === | === ETS-related flux control factors === | ||
* [[Biochemical coupling efficiency]]<ref>Gnaiger E. Biochemical coupling efficiency: from 0 to <1. Mitochondr Physiol Network. »[[Biochemical coupling efficiency]]«</ref> | ::::* [[Biochemical coupling efficiency]]<ref>Gnaiger E. Biochemical coupling efficiency: from 0 to <1. Mitochondr Physiol Network. »[[Biochemical coupling efficiency]]«</ref> | ||
* [[E-L coupling control factor]] - [[Excess E-P capacity factor]] - [[Excess E-R capacity factor]] | ::::* [[E-L coupling control factor]] - [[Excess E-P capacity factor]] - [[Excess E-R capacity factor]] | ||
=== ETS-related flux control ratios === | === ETS-related flux control ratios === | ||
* [[Coupling control ratio]] - [[Uncoupling control ratio]] | ::::* [[Coupling control ratio]] - [[Uncoupling control ratio]] | ||
* [[LEAK control ratio]] - [[OXPHOS control ratio]] | ::::* [[LEAK control ratio]] - [[OXPHOS control ratio]] | ||
* [[ROUTINE control ratio]] - [[NetROUTINE control ratio]] | ::::* [[ROUTINE control ratio]] - [[NetROUTINE control ratio]] | ||
== References == | |||
:::# Aragonés J, Schneider M, Van Geyte K, Fraisl P, Dresselaers T, Mazzone M, Dirkx R, Zacchigna S, Lemieux H, Jeoung NH, Lambrechts D, Bishop T, Lafuste P, Diez-Juan A, K Harten S, Van Noten P, De Bock K, Willam C, Tjwa M, Grosfeld A, Navet R, Moons L, Vandendriessche T, Deroose C, Wijeyekoon B, Nuyts J, Jordan B, Silasi-Mansat R, Lupu F, Dewerchin M, Pugh C, Salmon P, Mortelmans L, Gallez B, Gorus F, Buyse J, Sluse F, Harris RA, Gnaiger E, Hespel P, Van Hecke P, Schuit F, Van Veldhoven P, Ratcliffe P, Baes M, Maxwell P, Carmeliet P (2008) Deficiency or inhibition of oxygen sensor Phd1 induces hypoxia tolerance by reprogramming basal metabolism. Nat Genet 40:170-80. [[Aragones 2008 Nat Genet |»Open Access]]« | |||
:::# Gnaiger E. Biochemical coupling efficiency: from 0 to <1. Mitochondr Physiol Network. »[[Biochemical coupling efficiency]]« | |||
:::# Gnaiger E (2009) Capacity of oxidative phosphorylation in human skeletal muscle. New perspectives of mitochondrial physiology. Int J Biochem Cell Biol 41:1837-45. [[Gnaiger 2009 Int J Biochem Cell Biol |»PMID: 19467914]]« | |||
:::# Gnaiger E (2014) Mitochondrial pathways and respiratory control. An introduction to OXPHOS analysis. 4th ed. Mitochondr Physiol Network 19.12. OROBOROS MiPNet Publications, Innsbruck:80 pp. [[Gnaiger 2014 MitoPathways |»Open Access]]« | |||
:::# Hatefi Y, Haavik AG, Fowler LR, Griffiths DE (1962) Studies on the '''electron transfer system''' XLII. Reconstitution of the electron transfer system. J Biol Chem 237:2661-9. »[[Hatefi 1962 J Biol Chem-XLII |Open Access]]« | |||
:::# International Union of Biochemistry (1991) Nomenclature of '''electron-transfer proteins.''' Biochim Biophys Acta 1060. [http://www.chem.qmul.ac.uk/iubmb/etp/ »Open Access]« | |||
:::# International Union of Biochemistry and Molecular Biology. Recommendations for terminology and databases for biochemical thermodynamics - The IUPAC Green Book. [http://www.chem.qmul.ac.uk/iubmb/thermod2/ »Open Access]« | |||
:::# Pesta D, Hoppel F, Macek C, Messner H, Faulhaber M, Kobel C, Parson W, Burtscher M, Schocke M, Gnaiger E (2011) Similar qualitative and quantitative changes of mitochondrial respiration following strength and endurance training in normoxia and hypoxia in sedentary humans. Am J Physiol Regul Integr Comp Physiol 301:R1078–87. [[Pesta 2011 Am J Physiol Regul Integr Comp Physiol |»Open Access]]« | |||
::::* [[Bioblast_alert_2014#Bioblast_alert_2014.2804.29:_2014-07-07|Bioblast alert 2014(04)]] | |||
::::* [[Bioblast_alert_2013#Bioblast_alert_2013.2802.29:_2013-08-08|Bioblast alert 2013(02)]]: '''[[CCCP]]''' versus FCCP for high-resultion respirometry ([[HRR]]). | |||
* [[Bioblast_alert_2014#Bioblast_alert_2014.2804.29:_2014-07-07|Bioblast alert 2014(04)]] | |||
* [[Bioblast_alert_2013#Bioblast_alert_2013.2802.29:_2013-08-08|Bioblast alert 2013(02)]]: '''[[CCCP]]''' versus FCCP for high-resultion respirometry ([[HRR]]). | |||
Revision as of 16:21, 25 February 2016
Description
![]() ETS capacity is the respiratory electron transfer system capacity, E, of mitochondria in the experimentally induced noncoupled state. The conditions for measurement and expression of respiration vary (oxygen flux in state E, JO2E or oxygen flow in state E, IO2E). If these conditions are defined and remain consistent within a given context, then the simple symbol E for respiratory state is used to substitute the more explicit expression for respiratory activity. In state E, the mt-membrane potential is almost fully collapsed and provides a reference state for flux control ratios. In intact mitochondria, the ETS capacity depends not only on the inner membrane-bound ETS (mETS, with respiratory Complexes CI to CIV, electron-transferring flavoprotein ETF, and glycerophosphate dehydrogenase) but also integrates transporters across the inner mt-membrane, the TCA cycle and other matrix dehydrogenases. Its experimental determination in mitochondrial preparations or intact cells requires the measurement of oxygen consumption in the presence of defined substrates and of an established uncoupler at optimum concentration. This optimum concentration is determined by stepwise titration of the uncoupler up to the concentration inducing maximum flux.
» MiPNet article
ETS capacity is the respiratory electron transfer system capacity, E, of mitochondria in the experimentally induced noncoupled state. The conditions for measurement and expression of respiration vary (oxygen flux in state E, JO2E or oxygen flow in state E, IO2E). If these conditions are defined and remain consistent within a given context, then the simple symbol E for respiratory state is used to substitute the more explicit expression for respiratory activity. In state E, the mt-membrane potential is almost fully collapsed and provides a reference state for flux control ratios. In intact mitochondria, the ETS capacity depends not only on the inner membrane-bound ETS (mETS, with respiratory Complexes CI to CIV, electron-transferring flavoprotein ETF, and glycerophosphate dehydrogenase) but also integrates transporters across the inner mt-membrane, the TCA cycle and other matrix dehydrogenases. Its experimental determination in mitochondrial preparations or intact cells requires the measurement of oxygen consumption in the presence of defined substrates and of an established uncoupler at optimum concentration. This optimum concentration is determined by stepwise titration of the uncoupler up to the concentration inducing maximum flux.
» MiPNet article
Abbreviation: E
Reference: Gnaiger 2014 MitoPathways, Gnaiger 2009 Int J Biochem Cell Biol
MitoPedia concepts: Respiratory state
MitoPedia methods:
Respirometry
Why ETS, why not State 3u?
| Gnaiger E (2014) Why ETS, why not State 3u? Mitochondr Physiol Network 2014-07-06. |
Abstract: ![]() Measurement of ETS capacity in the noncoupled state at optimum uncoupler concentration does not represent a general substitute for determination of OXPHOS capacity (compare State 3). If the ratio of OXPHOS/ETS capacity (P/E ratio) is less than one, noncoupled respiration overestimates the apparent reserve capacity for oxidative phosphorylation with respect to ROUTINE respiration of intact cells.
Measurement of ETS capacity in the noncoupled state at optimum uncoupler concentration does not represent a general substitute for determination of OXPHOS capacity (compare State 3). If the ratio of OXPHOS/ETS capacity (P/E ratio) is less than one, noncoupled respiration overestimates the apparent reserve capacity for oxidative phosphorylation with respect to ROUTINE respiration of intact cells.
• O2k-Network Lab: AT Innsbruck Gnaiger E
Labels:
Coupling state: ETS"ETS" is not in the list (LEAK, ROUTINE, OXPHOS, ET) of allowed values for the "Coupling states" property.
HRR: Theory
Electron transfer system versus electron transport chain
- The well established terms 'respiratory chain' or 'electron transfer chain' suggest erroneously that the convergent electron transfer system may be designed as a simple chain. But the term electron transport chain (or electron transfer chain, ETC) is a misnomer. Understanding mitochondrial respiratory control has suffered greatly from this inappropriate terminology, although textbooks using the term ETC (Lehninger 1970) make it sufficiently clear that electron transfer systems are not arranged as a chain: the „ETC‟ is in fact not a simple chain but an arrangement of electron transfer complexes in a non-linear, convergent electron transfer system. The classically introduced term electron transfer system (Hatefi et al 1962) is accurate and sufficient (IUB 1991).
- The established convention of defining the 'electron transport chain' as being comprised of four Complexes has conceptual weaknesses.
- (a) In fact, there are at least six Complexes of mitochondrial electron transfer: In addition to Complexes I and II, glycerophosphate dehydrogenase complex (CGpDH) and electron-transferring flavoprotein complex (CETF) are involved in the Q-junction with electron transfer to Complex III (IUB 1991, Gnaiger 2014).
- (b) The term „chain‟ suggests a linear sequence, whereas the functional structure of the electron transfer system can only be understood by recognizing the convergence of electron flow at the Q-junction, followed by a chain of Complexes III and IV, mediated by cytochrome c (Gnaiger 2014).
- Electrons flow to oxygen from either Complex I with a total of three coupling sites, or from Complex II and other flavoproteins, providing multiple entries into the Q-cycle with two coupling sites downstream (Gnaiger 2014).
Electron transfer versus transport
- Electron transfer and electron transport are used synonymously. A general distinction, however, may be helpful:
- (i) Transfer (inter- or intramolecular) of a reactant involves a chemical reaction.
- (ii) Transport (from one place to another) of an entity is a (vectorial) process in contrast to a chemical reaction (IUPAC Green Book).
The important difference between states P and E
- The abbreviation State 3u is used frequently in bioenergetics, to indicate the noncoupled state of maximum respiration, E,[1] without sufficient emphasis on the fundamental difference between state P (OXPHOS capacity; coupled, with an uncoupled component; State 3) and state E (ETS capacity, noncoupled) (Gnaiger 2009, 2014).
- P=E: The specific case of equal OXPHOS and ETS capacity (P/E=1) yields the important information that the capacity of the phosphorylation system matches or is in potential excess of the ETS capacity, such that OXPHOS capacity is not limited by the phosphorylation system in the specific mitochondria. This varies with species and tissues, and changes as a result of pathologies due to defects in the phosphorylation system. An example for P/E=1 is mouse skeletal muscle mitochondria (Aragones et al 2008).
- P<E: When OXPHOS is less than ETS capacity, the phosphorylation system limits OXPHOS capacity, and there is an apparent ETS excess capacity. For example, this is the case in healthy human skeletal muscle mitochondria (Pesta et al 2011).
- P>E: If ETS is less than OXPHOS capacity in intact cells, or in mitochondrial preparations with defined substrate(s), then you have encountered an experimental artefact, and the apparent ETS capacity is too low. Artificially low ETS capacity may be obtained due to overtitration of uncoupler. Inhibitors of ATP synthase may suppress ETS capacity in intact cells, particularly in stressed cells.
Consequences for evaluation of coupling
- In some textbooks on Bioenergetics, the RCR is defined as either the State 3/State 4 ratio or the State 3u/State 4 ratio. This reflects lack of conceptual distinction between State 3 (or P) and 3u (E), and clarification is best achieved by avoiding ambiguous terminology. RCR as defined originally is the 'acceptor control ratio' or 'adenylate control ratio' (see LEAK control ratio, L/E; biochemical coupling efficiency). ETS capacity but not OXPHOS capacity provides a valid reference for an index of uncoupling.
Related terms in Bioblast
 OXPHOS, P
OXPHOS, P
 ROUTINE, R
ROUTINE, R
 ETS, E
ETS, E
 LEAK, L
LEAK, L
 ROX, R
ROX, R
The ETS state
References
- Aragonés J, Schneider M, Van Geyte K, Fraisl P, Dresselaers T, Mazzone M, Dirkx R, Zacchigna S, Lemieux H, Jeoung NH, Lambrechts D, Bishop T, Lafuste P, Diez-Juan A, K Harten S, Van Noten P, De Bock K, Willam C, Tjwa M, Grosfeld A, Navet R, Moons L, Vandendriessche T, Deroose C, Wijeyekoon B, Nuyts J, Jordan B, Silasi-Mansat R, Lupu F, Dewerchin M, Pugh C, Salmon P, Mortelmans L, Gallez B, Gorus F, Buyse J, Sluse F, Harris RA, Gnaiger E, Hespel P, Van Hecke P, Schuit F, Van Veldhoven P, Ratcliffe P, Baes M, Maxwell P, Carmeliet P (2008) Deficiency or inhibition of oxygen sensor Phd1 induces hypoxia tolerance by reprogramming basal metabolism. Nat Genet 40:170-80. »Open Access«
- Gnaiger E. Biochemical coupling efficiency: from 0 to <1. Mitochondr Physiol Network. »Biochemical coupling efficiency«
- Gnaiger E (2009) Capacity of oxidative phosphorylation in human skeletal muscle. New perspectives of mitochondrial physiology. Int J Biochem Cell Biol 41:1837-45. »PMID: 19467914«
- Gnaiger E (2014) Mitochondrial pathways and respiratory control. An introduction to OXPHOS analysis. 4th ed. Mitochondr Physiol Network 19.12. OROBOROS MiPNet Publications, Innsbruck:80 pp. »Open Access«
- Hatefi Y, Haavik AG, Fowler LR, Griffiths DE (1962) Studies on the electron transfer system XLII. Reconstitution of the electron transfer system. J Biol Chem 237:2661-9. »Open Access«
- International Union of Biochemistry (1991) Nomenclature of electron-transfer proteins. Biochim Biophys Acta 1060. »Open Access«
- International Union of Biochemistry and Molecular Biology. Recommendations for terminology and databases for biochemical thermodynamics - The IUPAC Green Book. »Open Access«
- Pesta D, Hoppel F, Macek C, Messner H, Faulhaber M, Kobel C, Parson W, Burtscher M, Schocke M, Gnaiger E (2011) Similar qualitative and quantitative changes of mitochondrial respiration following strength and endurance training in normoxia and hypoxia in sedentary humans. Am J Physiol Regul Integr Comp Physiol 301:R1078–87. »Open Access«
- Bioblast alert 2014(04)
- Bioblast alert 2013(02): CCCP versus FCCP for high-resultion respirometry (HRR).
List of publications: ETS
- ↑ Gnaiger E. Electron transfer system versus electron transport chain. Mitochondr Physiol Network. »Electron transfer system«
- ↑ Gnaiger E. Is respiration uncoupled - noncoupled - dyscoupled? Mitochondr Physiol Network. »Uncoupler«
- ↑ Gnaiger E. Biochemical coupling efficiency: from 0 to <1. Mitochondr Physiol Network. »Biochemical coupling efficiency«